A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-EQUILIBRIUM -Assertion And Reason
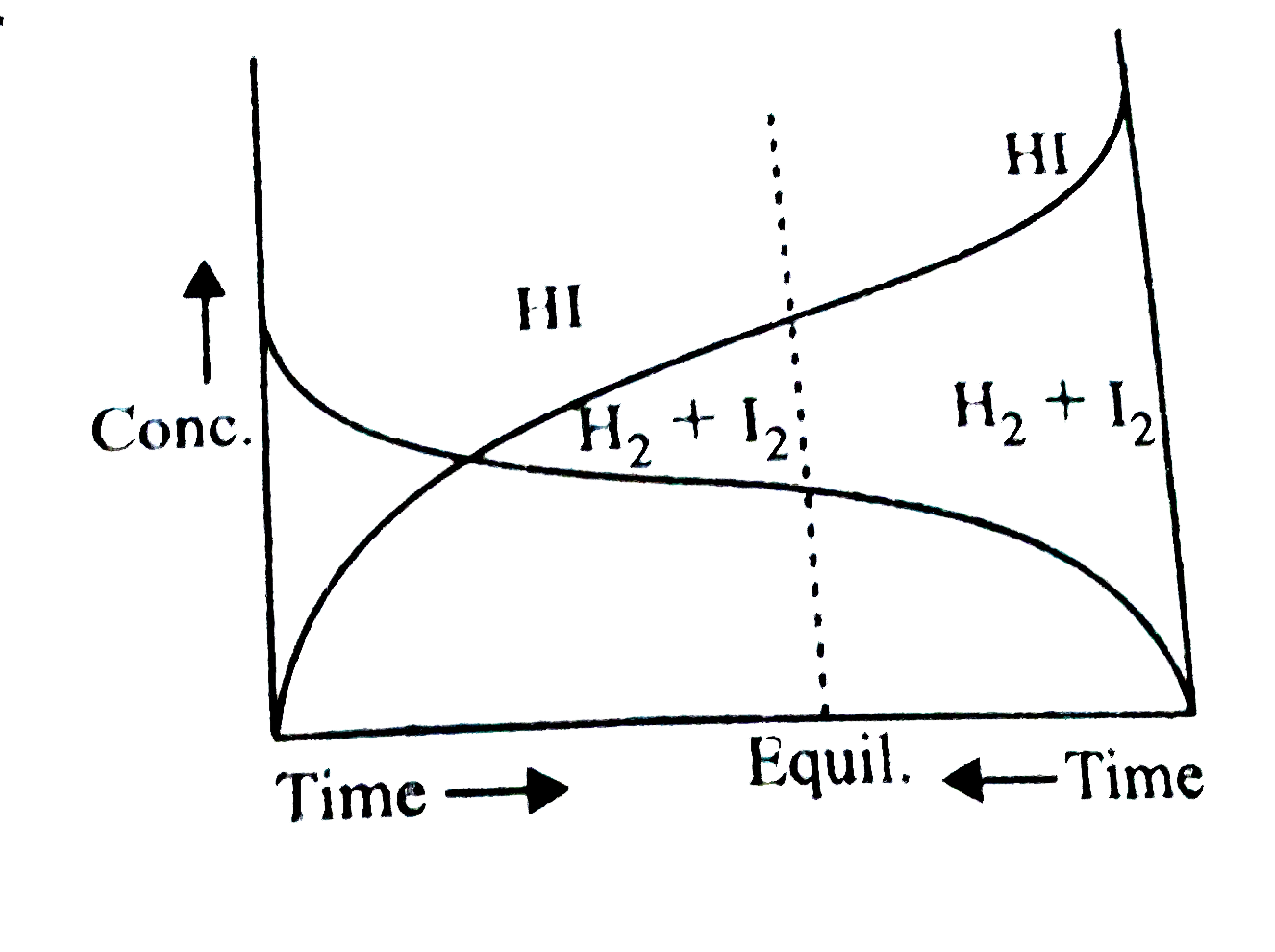
- Consider the following graph and mark the correct statement.
Text Solution
|
- Assertion : When ice and water are kept in a perfectly insulated therm...
Text Solution
|
- Assertion : The equilibrium constant for the reverse reaction is equa...
Text Solution
|
- Assertion : For the reaction : N(2(g))+3H(2(g))hArr2NH(3(g)),K(p)=K(c)...
Text Solution
|
- Assertion : K(p) can be less than, greater than or equal to K(c) Rea...
Text Solution
|
- Assertion : If reaction quotient, Q(c) for a particular reaction is gr...
Text Solution
|
- Assertion : In the dissociation of PCl(5) at constant pressure and tem...
Text Solution
|
- Assertion : Weak acids have very strong conjugate bases while strong a...
Text Solution
|
- Assertion :- A solution of NH(4)Cl in water is acidic in nature. ...
Text Solution
|
- Statement: The pH of an aqueous solution of acetic acid remains unchan...
Text Solution
|
- Assertion : Higher order ionization constants (K(a(2)),K(a(3))) are sm...
Text Solution
|
- Assertion : Benzoic acid is stronger acid than acetic acid. Reason ...
Text Solution
|
- Assertion : The strength of haloacids increases in the order : HIltltH...
Text Solution
|
- Assertion : The pH of NH(4)Cl solution in water is less than 7 and pH ...
Text Solution
|
- Assertion : pH of the buffer solution is not affected by dilution. ...
Text Solution
|
- Assertion : The solubility of salts of weak acids like phosphates decr...
Text Solution
|