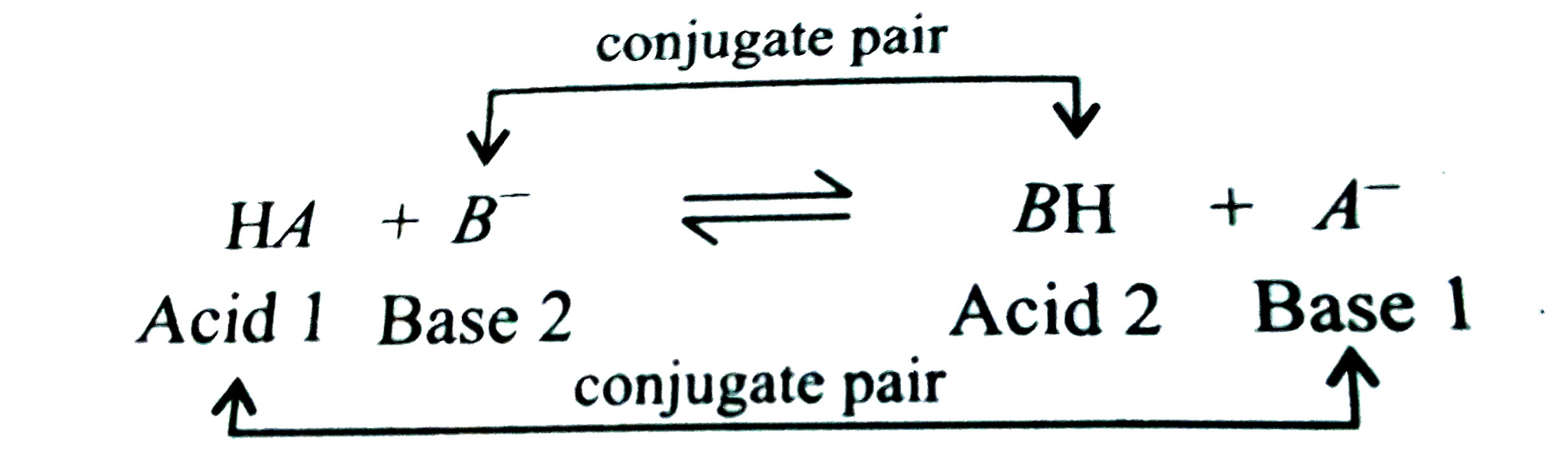A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
NCERT FINGERTIPS ENGLISH|Exercise Ionization Of Acids And Bases|19 VideosEQUILIBRIUM
NCERT FINGERTIPS ENGLISH|Exercise Buffer Solutions|2 VideosEQUILIBRIUM
NCERT FINGERTIPS ENGLISH|Exercise Factors Affecting Equilibria|10 VideosENVIRONMENTAL CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosHYDROCARBONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-EQUILIBRIUM -Acids, Bases And Salts
- According to Lewis concept acid is(A) proton donor (B)electron pair do...
Text Solution
|
- Which of thef following is not Lewis acid
Text Solution
|
- Conjugate acid of SO(4)^(2-) is
Text Solution
|
- Which of the following species can act both as an acid as well as a ba...
Text Solution
|
- According to Bronsted - Lowry concept of acids and bases a conjugate a...
Text Solution
|
- Classify the following as acid or base according to Bronsted - Lowry c...
Text Solution
|
- Nucleophiles are while electrophiles are .
Text Solution
|
- Which of the following salts will give basic solution on hydrolysis ?
Text Solution
|
- Which of the following salts with a concentration .1 M will give a bas...
Text Solution
|