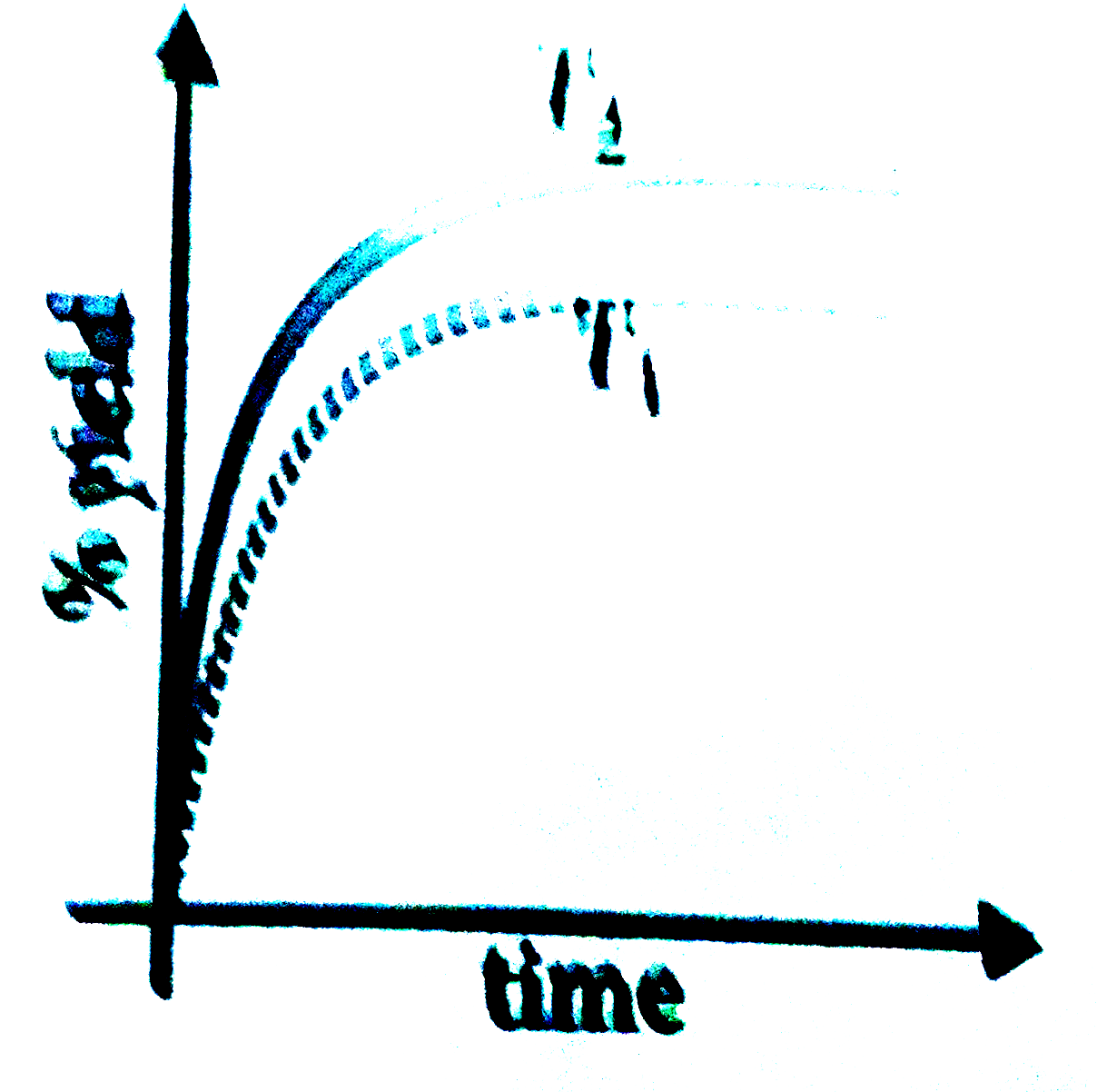A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|19 VideosEQUILIBRIUM
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosEQUILIBRIUM
NCERT FINGERTIPS ENGLISH|Exercise Solubility Equilibria Of Sparingly Soluble Solids|15 VideosENVIRONMENTAL CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosHYDROCARBONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-EQUILIBRIUM -Higher Order Thinking Skills
- At 1127 K and 1 atm pressure, a gaseous mixture of CO and CO(2) in equ...
Text Solution
|
- A mixture of 1.57 mol of N(2), 1.92 mol of H(2) and 8.13 mol of NH(3) ...
Text Solution
|
- N(2)O(4) hArr 2NO(2), K(c)=4. This reversible reaction is studied grap...
Text Solution
|
- At 473K, equilibrium constant, K(c) for decomposition of phosphorus pe...
Text Solution
|
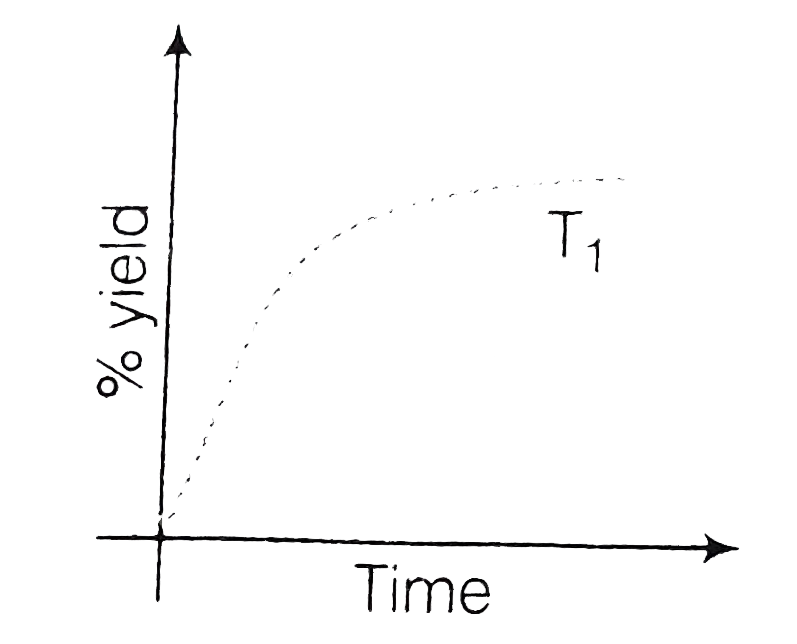
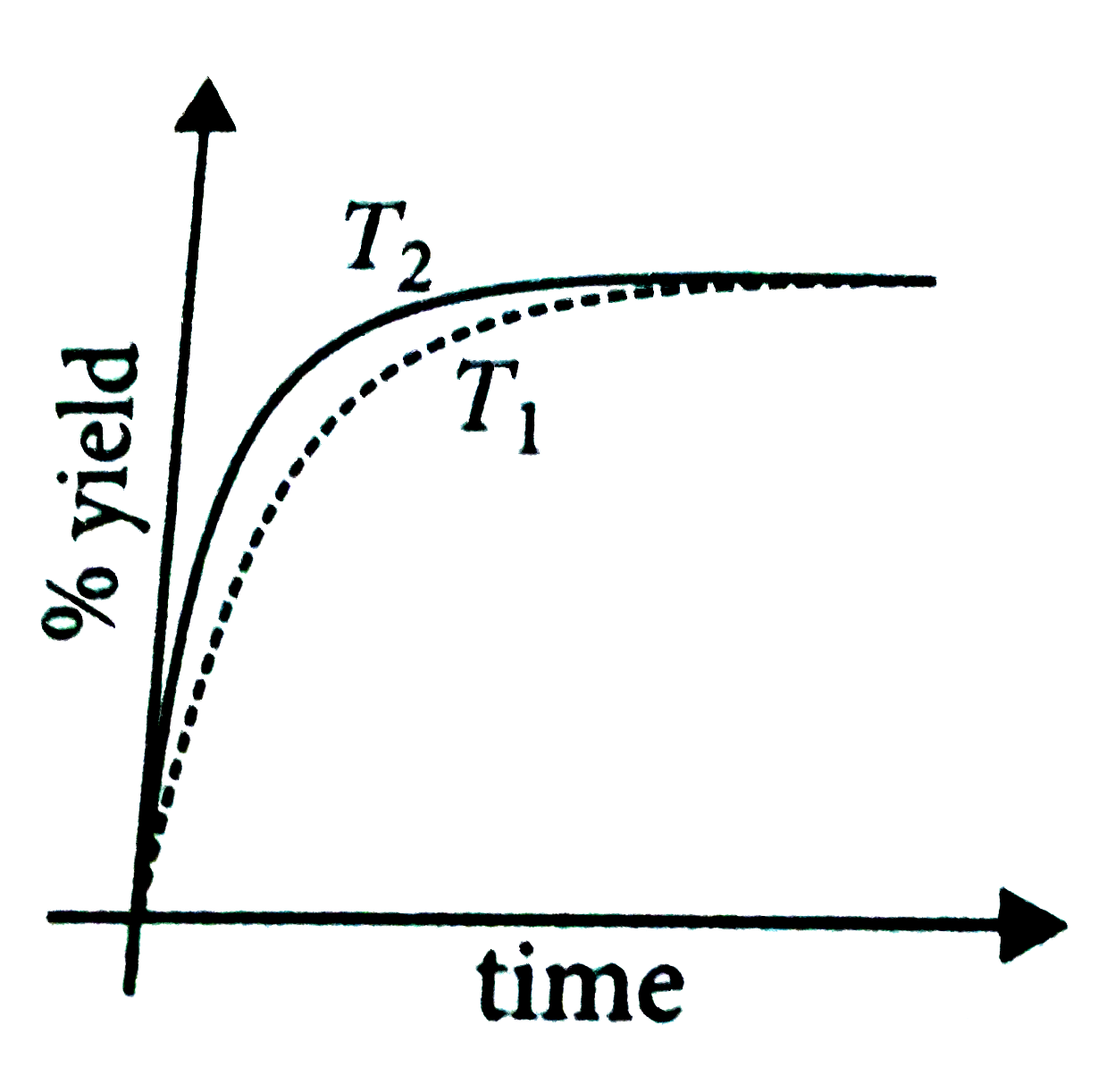
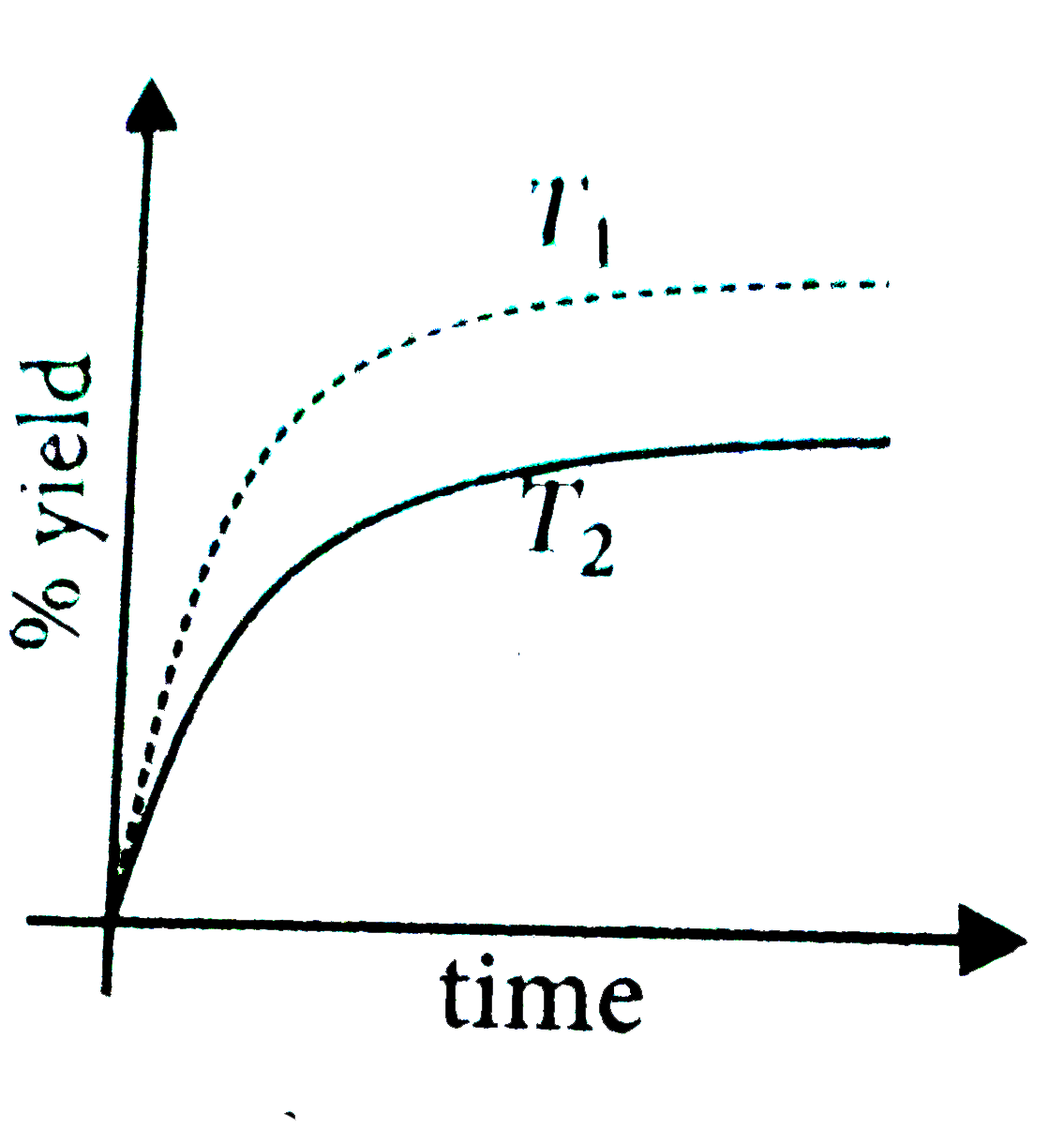
- The % yield of ammonia as a function of time in the reaction, N(2)(g)+...
Text Solution
|
- The ionisation constant of benzoic acid (PhCOOH) is 6.46 xx 10^(-5) an...
Text Solution
|
- A solution which is 10^(-3) M each in Mn^(2+),Fe^(2+),Zn^(2+)andHg^(2+...
Text Solution
|
- What will be the amount of (NH(4))(2)SO(4) (in g) which must be added ...
Text Solution
|