A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
NCERT FINGERTIPS ENGLISH|Exercise MCQs (REDOX REACTIONS AND ELECTRODE PROCESSES)|22 VideosREDOX REACTIONS
NCERT FINGERTIPS ENGLISH|Exercise HIGHER ORDER THINKING SKILLS|9 VideosREDOX REACTIONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosPRACTICE PAPER 3
NCERT FINGERTIPS ENGLISH|Exercise Practice Paper 3|46 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS ENGLISH|Exercise NCERT Exemplar|11 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-REDOX REACTIONS-MCQs (OXIDATION NUMBER)
- In which of the following compounds oxidation state of chlorine has tw...
Text Solution
|
- The oxidation number of nitrogen in (N(2)H(5))^(+) is
Text Solution
|
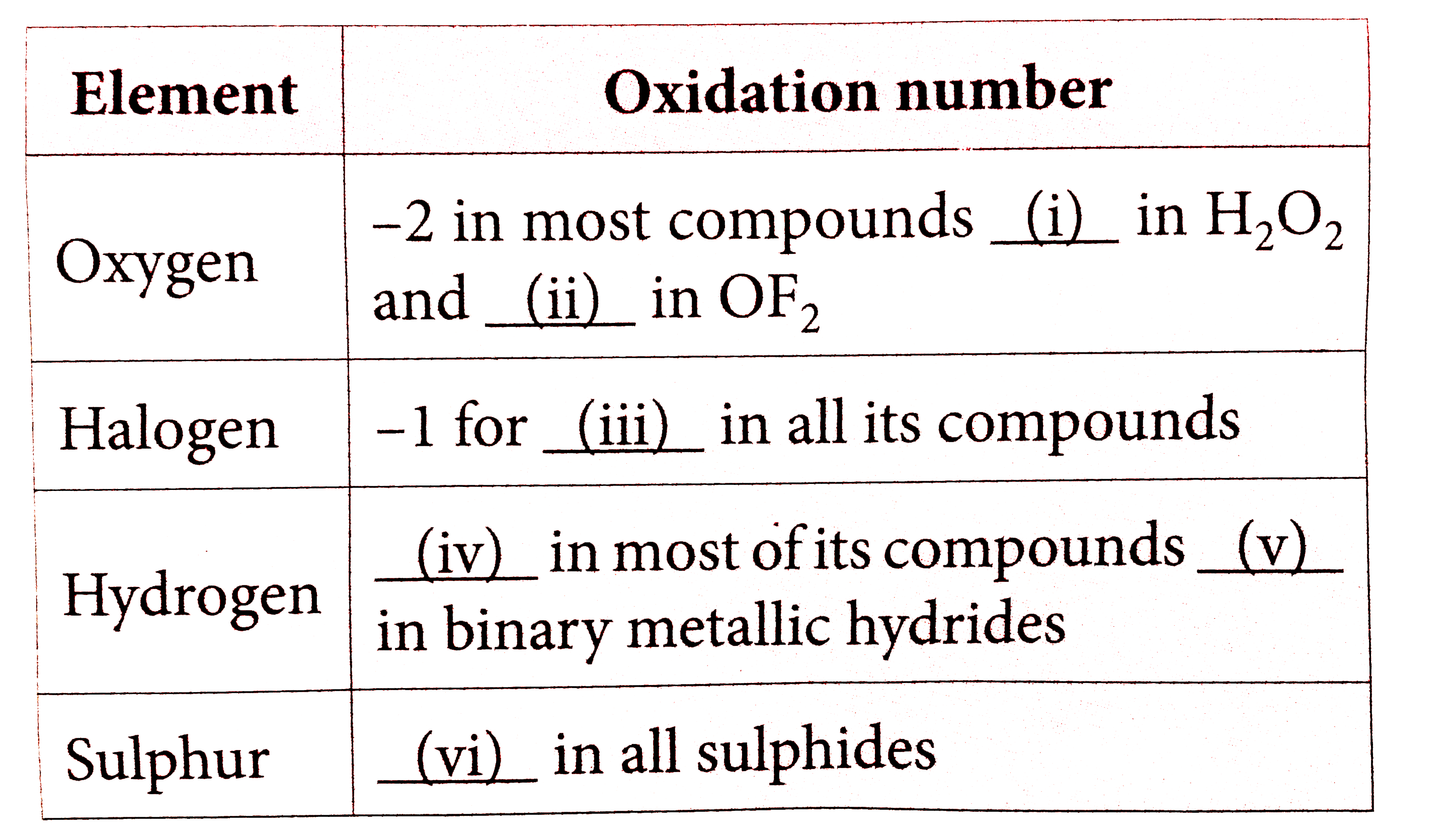
- Fill up the table from the given choice.
Text Solution
|
- Mark the correct statement from the following :
Text Solution
|
- Which compound among the following has lowest oxidation number of chlo...
Text Solution
|
- Which of the following oxidation numbers is not correctly matched ?
Text Solution
|
- Examples of few compounds in a particular oxidation state are given. ...
Text Solution
|
- The oxidation numbers of the sulphur atoms in pcroxy- monosulphuric ac...
Text Solution
|
- Which is not true about the oxidation state of the following elements ...
Text Solution
|
- O.N. (Oxidation Number) of Fe in K(4)[Fe(CN)(6)] is
Text Solution
|
- Arrange the following in increasing order of oxidation state of Ni. ...
Text Solution
|
- The correct sequence of the oxidation state of underlined elements is ...
Text Solution
|
- What are the oxidation states of phosphorus in the following compounds...
Text Solution
|
- In which of the following compounds carbon is in highest oxidation sta...
Text Solution
|
- The oxidising state of molybdenum in its oxo complex species [Mo(2)O...
Text Solution
|
- Oxidation number of P in Ba(H(2)PO(2))(2) is
Text Solution
|
- Which of the following can act as oxidising as well as reducing agent?
Text Solution
|
- When a piece of sodium metal is dropped in water, hydrogen gas evolved...
Text Solution
|
- In the reaction, I(2)+2S(2)O(3)^(2-) rarr 2I^(-)+S(4)O(6)^(2-).
Text Solution
|
- In the reaction :Cl(2)+OH^(-)rarrCl^(-)+ClO(4)^(-)+H(2)O :-
Text Solution
|