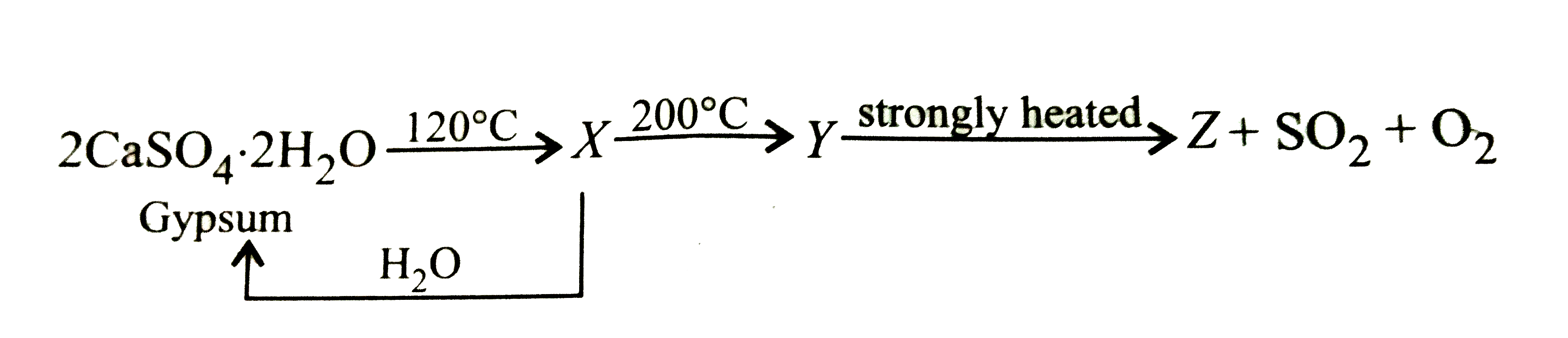A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE S-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Higher Order Thinkin Skills|10 VideosTHE S-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Exemplar Problems|21 VideosTHE P-BLOCK ELEMENTS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosTHERMODYNAMICS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-THE S-BLOCK ELEMENTS-Assertion And Reason
- Identify X, Y and Z
Text Solution
|
- Assertion: Elements of group 1 are called 'alkali metals'. Reason: A...
Text Solution
|
- Assertion: Lithium resembles magnesium diagonally placed in next group...
Text Solution
|
- Assertion: Alkali metals are obtained by electrolysis of molten salt a...
Text Solution
|
- Assertion: Lithium salts are mostly hydrated. Reason: The hydration ...
Text Solution
|
- Assertion: The melting and boiling points of the alkali metals are low...
Text Solution
|
- Assertion: Lithium fluoride is most covalent in nature. Reason: Smal...
Text Solution
|
- Assertion: The carbonate of lithium decomposes easily on heating. Re...
Text Solution
|
- Assertion : Super-oxides of alkali metals are para-magnetic. Reason ...
Text Solution
|
- Assertion: Be and Mg do not impat characteristic colour to the flame. ...
Text Solution
|
- Assertion: The fluorides of alkaline earth metals are relatively less ...
Text Solution
|
- Assertion: Be is readily attacked by acids. Reason: Be shows diagona...
Text Solution
|
- Assertion: Alkaline earth metal oxides are quite stable to heat. Rea...
Text Solution
|
- Assertion: BeSO(4) and MgSO(4) are insoluble in water. Reason: Be^(2...
Text Solution
|
- Assertion: CaCO(3) is prepared by passing carbon dioxide gas through s...
Text Solution
|
- Assertion: For biological functions in human body, barium is not requi...
Text Solution
|