A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-PRACTICE PAPER 3-Practice Paper 3
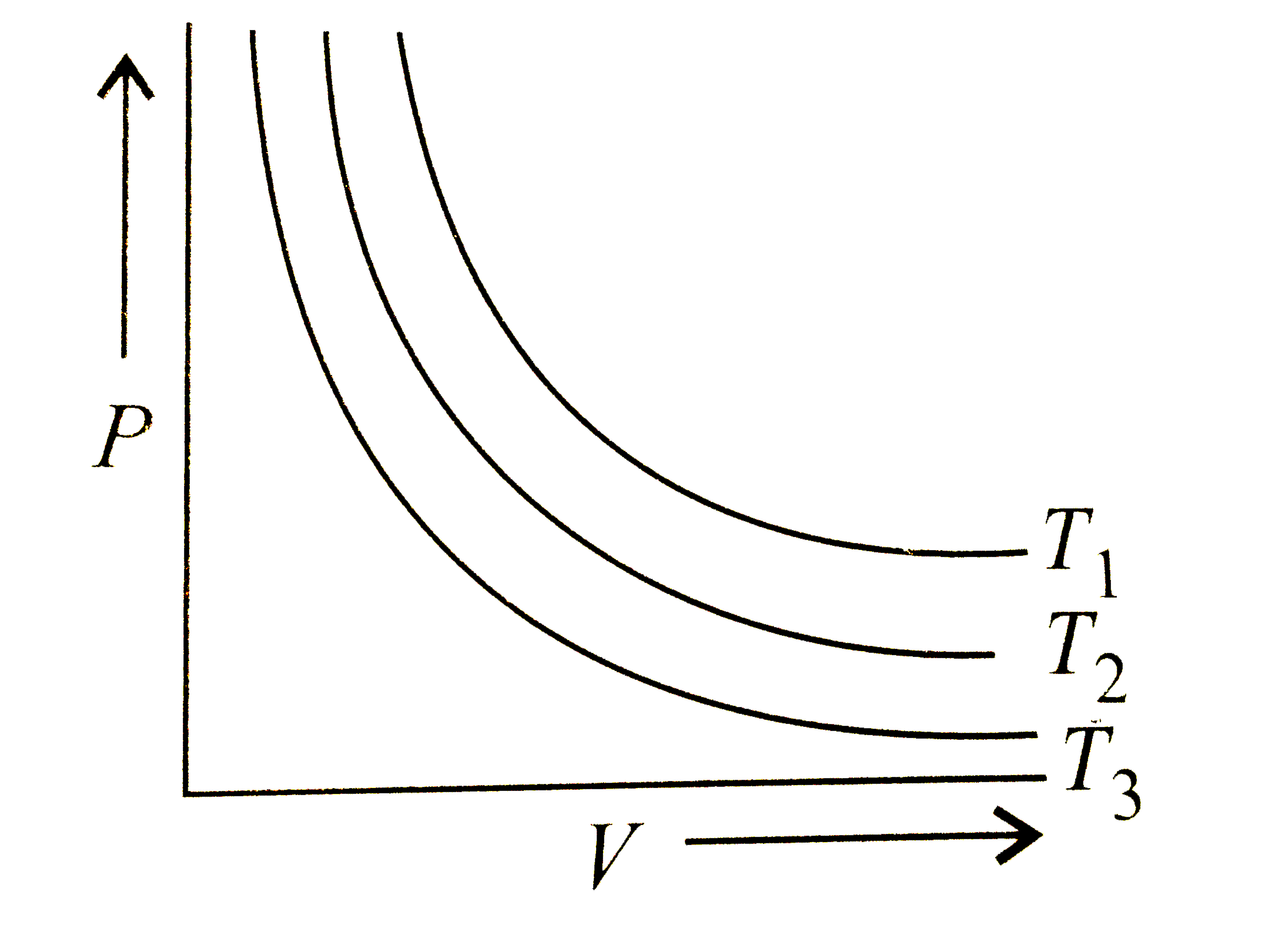
- The graph of P vs V is given at different temperature The correct...
Text Solution
|
- Compressibility factor for H(2) behaving as real gas is
Text Solution
|
- Which of the following statements is correct with respect to the prope...
Text Solution
|
- A sample of calcium carbonate (CaCO(3)) has the following percentage c...
Text Solution
|
- For the reaction, CO((g))+Cl(2(g))hArrCoCl(2(g)), the value of K(p)//K...
Text Solution
|
- Which of the following is not a basic physical quantity?
Text Solution
|
- In any subshell, the maximum number of electrons having same value of ...
Text Solution
|
- Clean water would have BOD value of less than
Text Solution
|
- Which of the followin will show least dipole character?
Text Solution
|
- Indicate the wrongly named compound.
Text Solution
|
- Heavy water is used as a
Text Solution
|
- The pH of 0.05 M Ba(OH)(2) solution is
Text Solution
|
- Which of the following solutions will have pH close to 1.0?
Text Solution
|
- The signs of DeltaH,DeltaS and DeltaG for a non-spontaneous reaction a...
Text Solution
|
- Which oxide is formed when potassium is heated in e3xcess of oxygen?
Text Solution
|
- What is the decreasing order of strength of the bases OH^(-),NH(2)^(-)...
Text Solution
|
- The electrons, identified by quantum numbers n and l(i) n=4,l=1 (ii) n...
Text Solution
|
- Conjugate base of a strong acid is
Text Solution
|
- A sample of gas has a volume of V(1) litre at temperature t(1).^(@)C. ...
Text Solution
|
- The liquefaction behaviour of temporary gases approacches that of perr...
Text Solution
|
- When the temperature is raised, viscosity o the liquid decreases. This...
Text Solution
|