A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
NCERT FINGERTIPS ENGLISH|Exercise Higher Order Thinkin Skills|7 VideosHYDROCARBONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion & Reason|15 VideosEQUILIBRIUM
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosHYDROGEN
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-HYDROCARBONS -Assertion And Reason
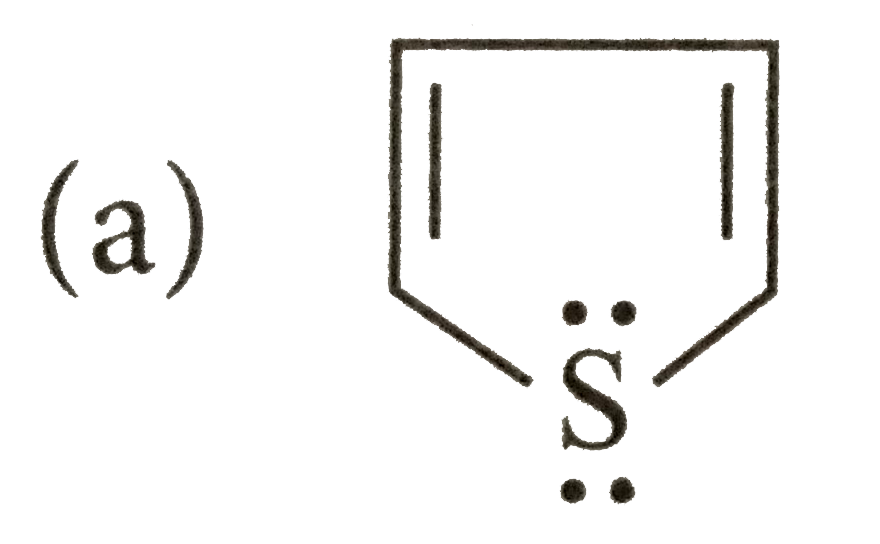
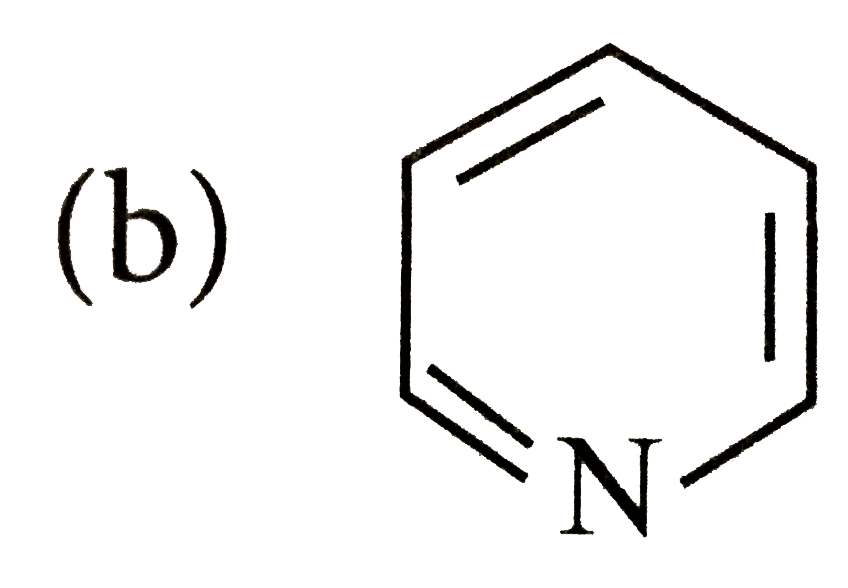
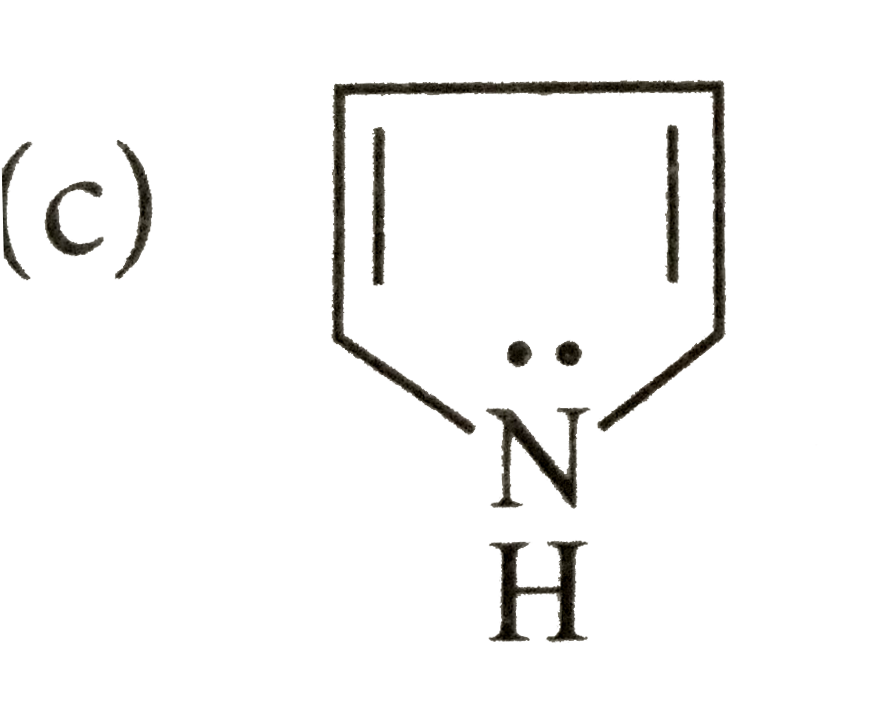
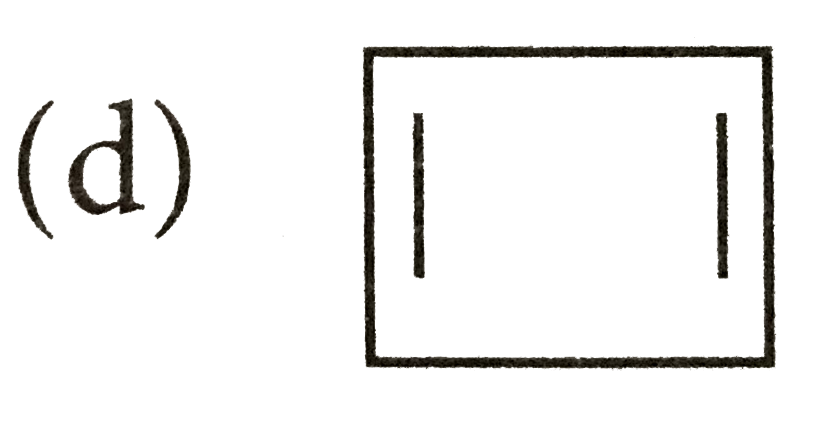
- Which of the following species does not show aromaticity?
Text Solution
|
- Assertion: 2,2-Dimethylbutane does not have any tertiary carbon atom. ...
Text Solution
|
- Assertion: The reaction, C(2)H(5)Br +2Na+C(2)H(5)Br to C(4)H(10)+2NaBr...
Text Solution
|
- Assertion: Wurtz reaction is not preferred for the preparation of alka...
Text Solution
|
- Assertion: Sodium salt of butanoic acid on heating with soda lime give...
Text Solution
|
- Assertion: Boiling point of pentane is higher than 2,2-dimethylpropane...
Text Solution
|
- Assertion: Iodination of alkanes is carried out in the presence of oxi...
Text Solution
|
- Assertion: Staggered conformation of ethane is most stable while eclip...
Text Solution
|
- Assertion: cis-form of alkene is found to be more polar than the trans...
Text Solution
|
- Assertion: Alkenes are easily attacked by electrophilic reagents. Re...
Text Solution
|
- Assertion: Addition of HBr to propene yields 2-bromopropane but in pre...
Text Solution
|
- Assertion: Decolourisation of KMnO(4) solution is used as a test for u...
Text Solution
|
- Assertion: Ethyne reacts with sodium metal and sodamide to form sodium...
Text Solution
|
- Assertion: Cyclopentadienyl anion is aromatic in nature. Reason: Cyc...
Text Solution
|
- Assertion: The second substituent may enter the mono-substituted benze...
Text Solution
|
- Assertion: In case of aryl halides, halogens are moderately deactivati...
Text Solution
|