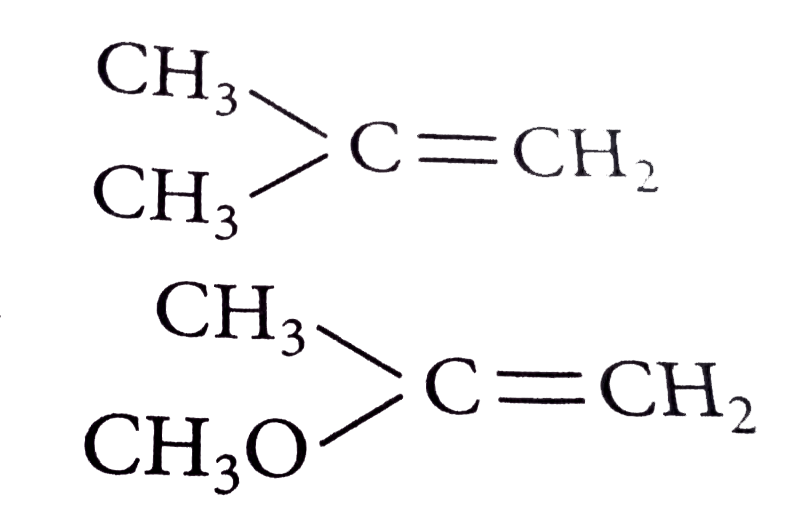
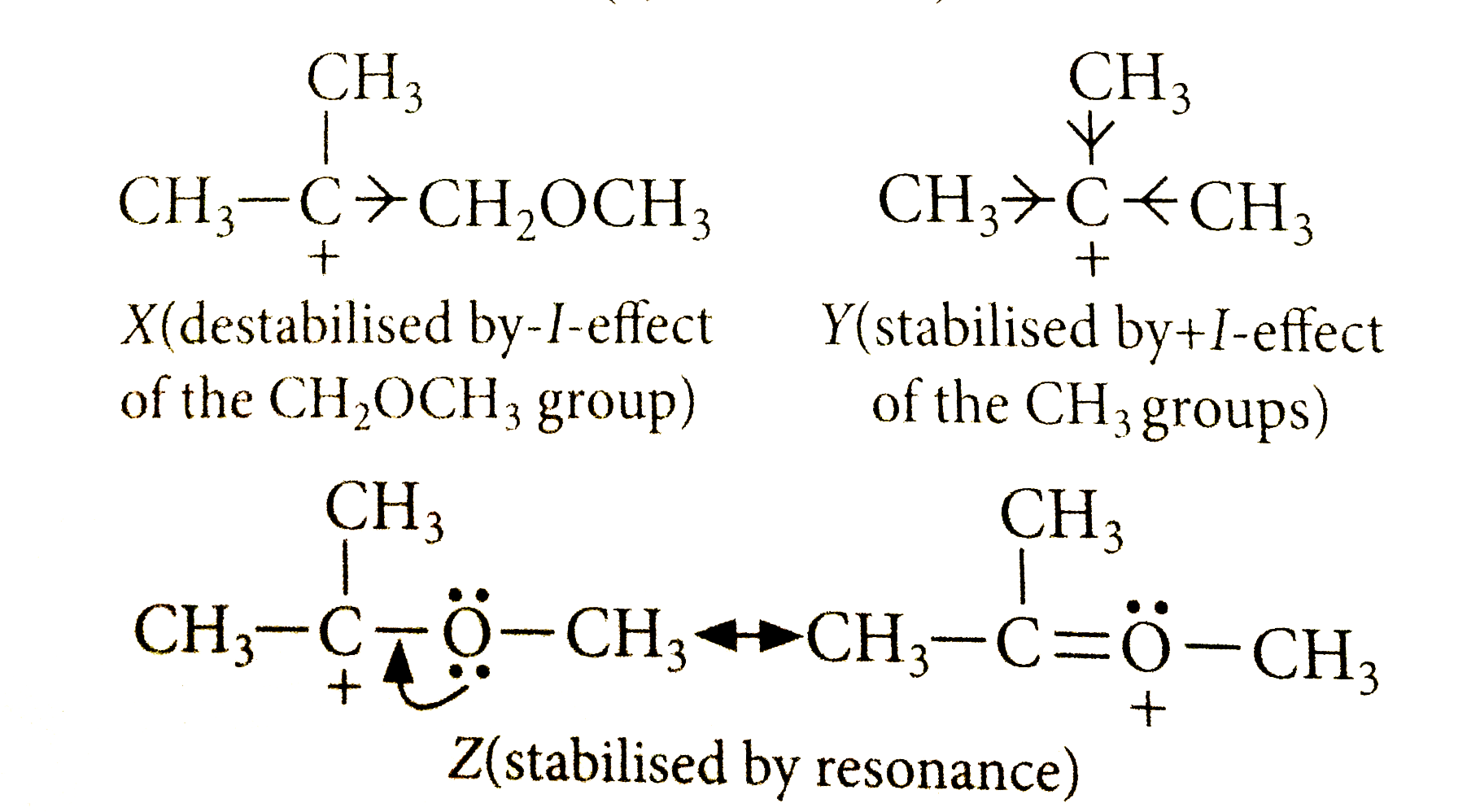
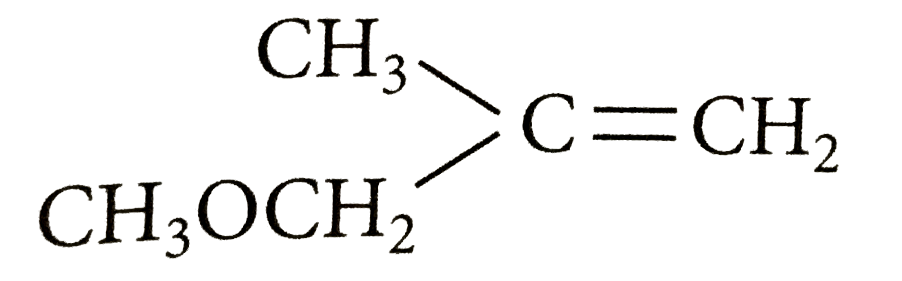A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion & Reason|15 VideosHYDROCARBONS
NCERT FINGERTIPS ENGLISH|Exercise Alkanes|25 VideosHYDROCARBONS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosEQUILIBRIUM
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 VideosHYDROGEN
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-HYDROCARBONS -Higher Order Thinkin Skills
- 1-Bromo-3-chlorocyclobutane is treated with two equivalents of Na, in ...
Text Solution
|
- CH(3)CH(2)CH(2)CH(3) underset(hv)overset(Cl(2))to underset(("monochlor...
Text Solution
|
- Observe the following reactions and predict the products A and B
Text Solution
|
- Arrange the following alkenes in decreasing order of their rea...
Text Solution
|
- Which of the following will exhibit aromatic character?
Text Solution
|
- Consider the following compounds: Which compound possesses highes...
Text Solution
|
- Consider the following compounds: the correct order towards elect...
Text Solution
|