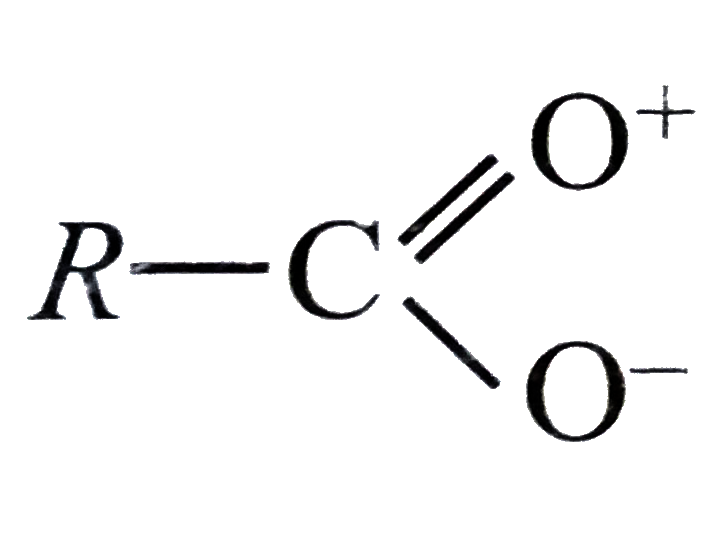
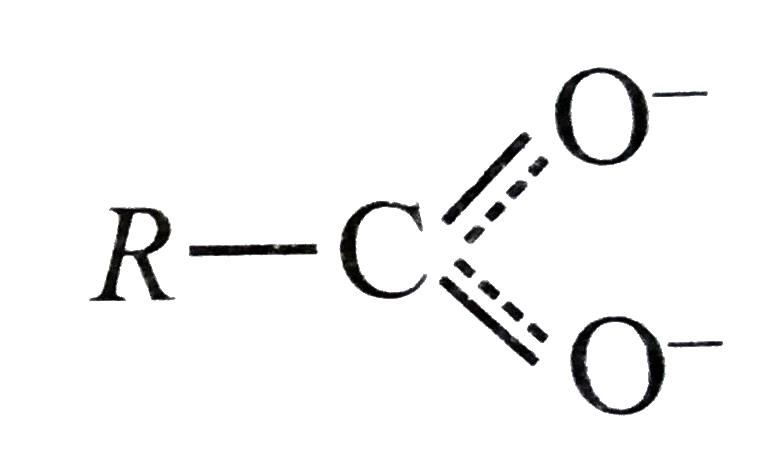
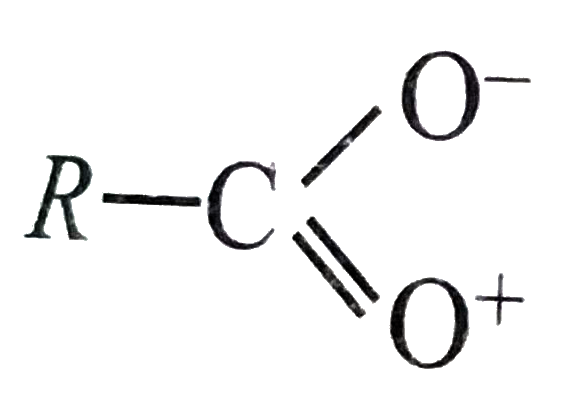
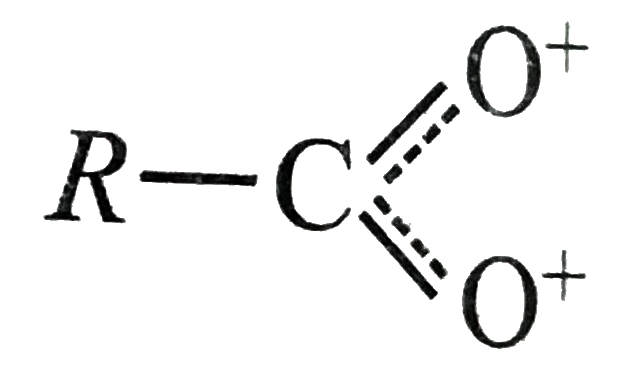
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
NCERT FINGERTIPS ENGLISH|Exercise MCQs( METHODS OF PREPARATION OF CARBOXYLIC ACIDS )|7 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
NCERT FINGERTIPS ENGLISH|Exercise MCQs( PHYSICAL PROPERTIES )|2 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
NCERT FINGERTIPS ENGLISH|Exercise MCQs( CHEMICAL REACTIONS )|50 VideosALCOHOLS,PHENOLS AND ETHERS
NCERT FINGERTIPS ENGLISH|Exercise Assertion And Reason|14 VideosAMINES
NCERT FINGERTIPS ENGLISH|Exercise ASSERTION & REASON CORNER|14 Videos
Similar Questions
Explore conceptually related problems