A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS ENGLISH-PRACTICE PAPER -1-Practice Paper 1
- The element with atomic number 33 belongs to
Text Solution
|
- For H(3)PO(3) and H(3)PO(4) the correct choice is
Text Solution
|
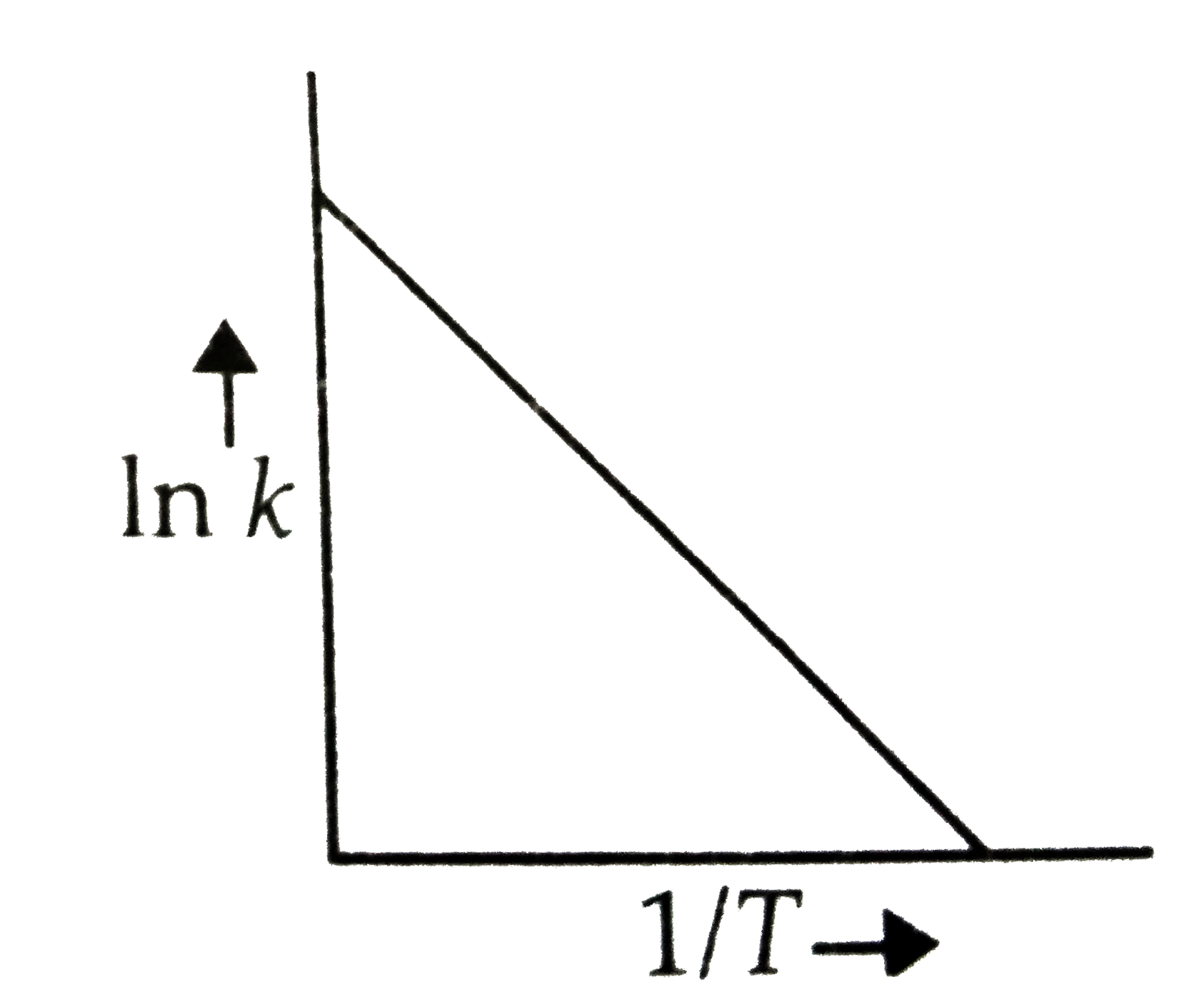
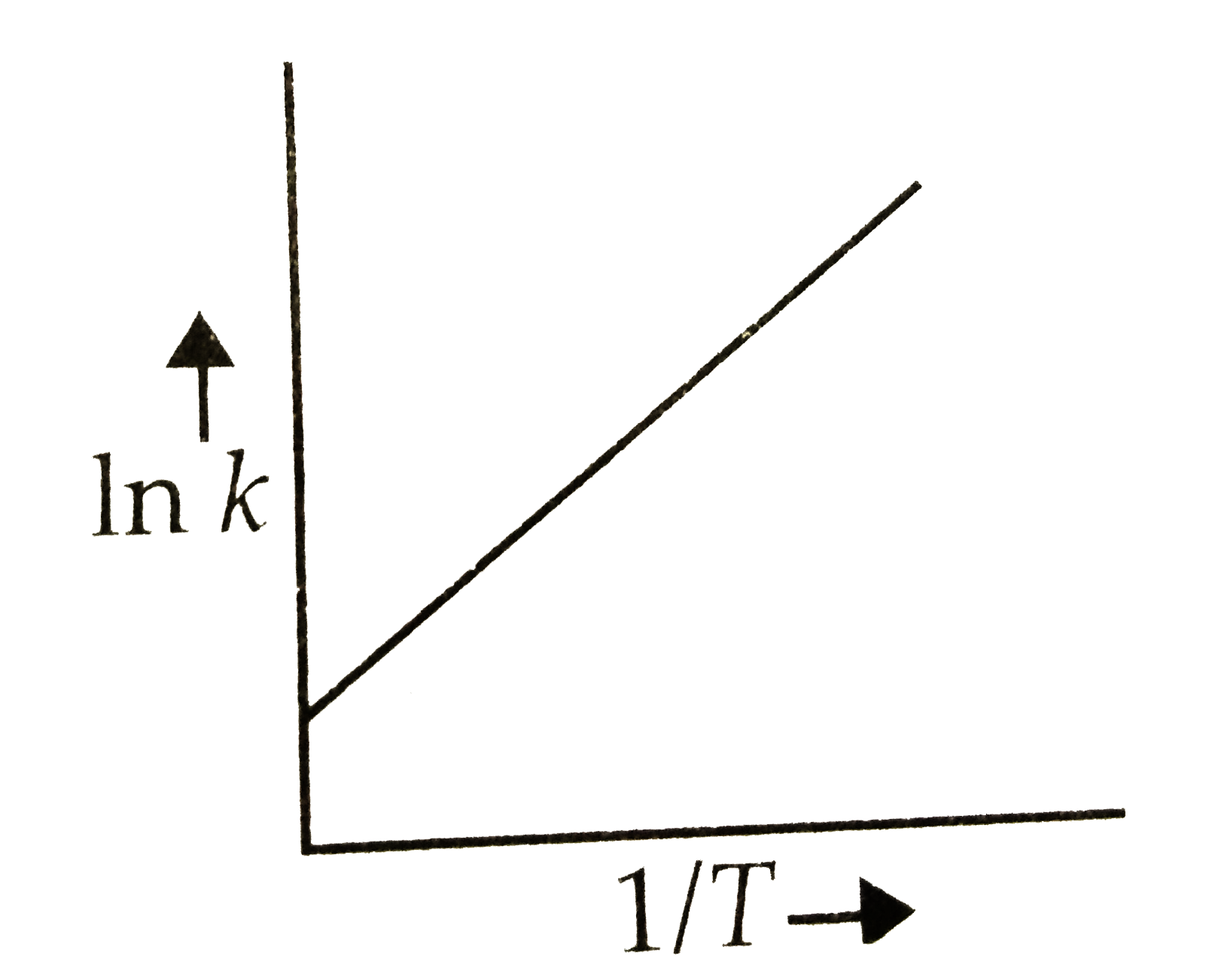
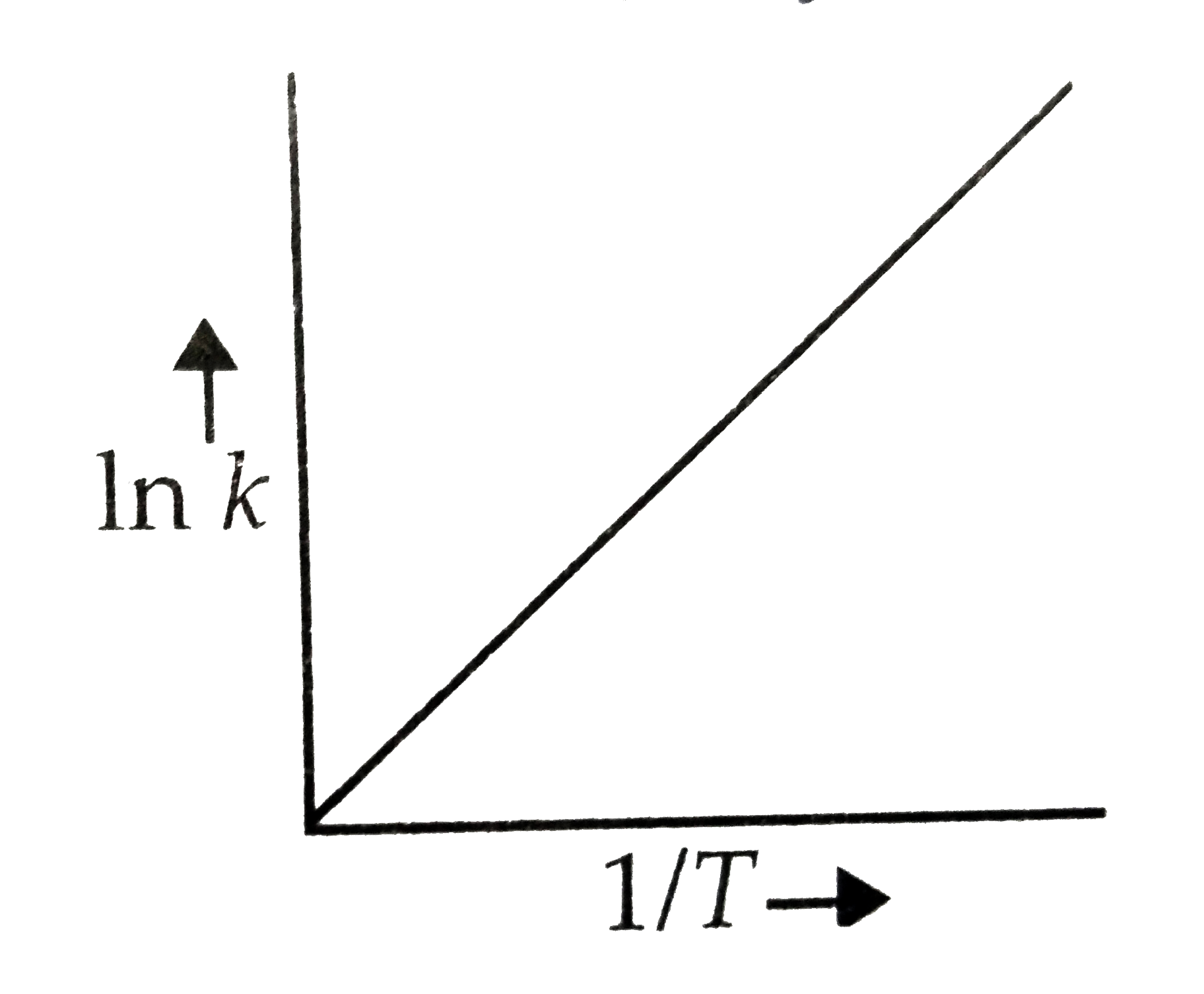
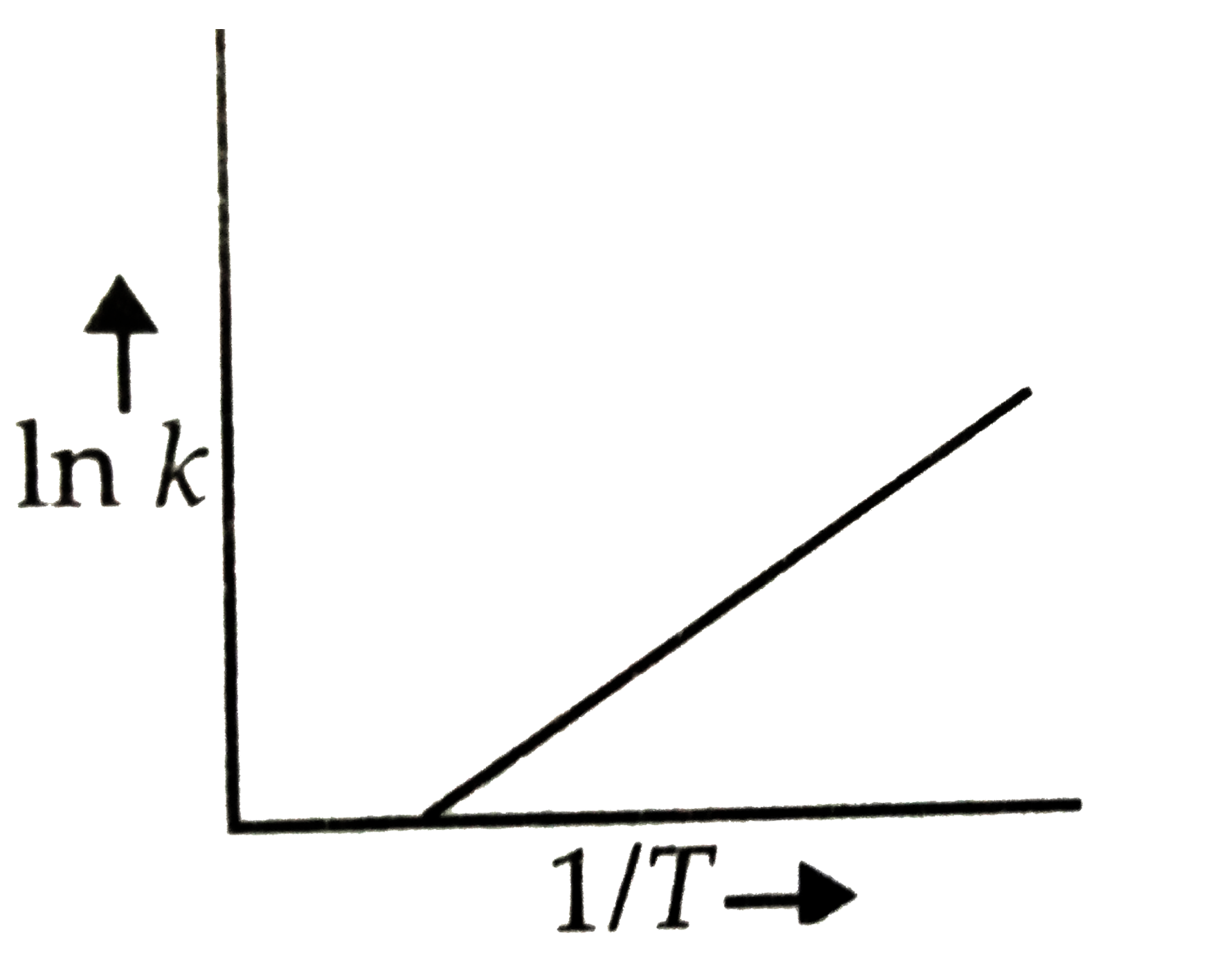
- According to Arrhenius equation rate constant k is equal to Ae^(-E(a)/...
Text Solution
|
- Among the electrolytes Na(2)SO(4) , CaCI(2), AI(2)(SO(4))(3) and NH(4...
Text Solution
|
- Which is correct about saccharin ?
Text Solution
|
- In which mode of expression the concentration of solution remains inde...
Text Solution
|
- What is X ?
Text Solution
|
- The order of reactivities of the following alkyl halides for a S(N^(2)...
Text Solution
|
- Gabriel phthalimide reaction is used for the preparation of amines
Text Solution
|
- CH(3)CHO+HCHOoverset("dil. NaOH")underset("Heat")rarrAoverset("HCN)und...
Text Solution
|
- Drugs which bind strongly to the active site of an enzyme and do no...
Text Solution
|
- Tincture of iodine is
Text Solution
|
- Which of the following cannot show linkage isomerism?
Text Solution
|
- The oxidation of central atom in the complex [ Co(NH(3))(4) CINO(2)] ...
Text Solution
|
- Which of the following are intermediates in the reaction of excess of ...
Text Solution
|
- Acetic acid is obtained when
Text Solution
|
- In alpha -helix , structure, polypepetide chains are folded in a
Text Solution
|
- The electrode potential is the tendency of metal
Text Solution
|
- Transition elements exhibit higher enthalpiese of atomization because
Text Solution
|
- Which of the following is a monomer of natural rubber ?
Text Solution
|