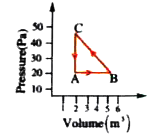
Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
AAKASH SERIES|Exercise TEST YOUR SELF|17 VideosTHERMODYNAMICS
AAKASH SERIES|Exercise SHORT ANSWER TYPE QUESTIONS|8 VideosTHERMODYNAMICS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET ((ADVANCED) INTEGER/SUBJECTIVE TYPE QUESTIONS))|11 VideosTHERMAL PROPERTIES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II) PRACTICE SHEET (ADVANCED) Integer/Subjective Type Questions|2 VideosTHERMOMETRY
AAKASH SERIES|Exercise Numerical Exercise (LEVEL- 1)|11 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-THERMODYNAMICS-PROBLEMS
- A fluid is contained in a cylinder by a spring loaded, frictionless pi...
Text Solution
|
- An ideal gas is taken through a cyclic thermodynamic process though fo...
Text Solution
|
- Three moles of an ideal gas at 300 K are isothermally expanded to five...
Text Solution
|
- The pressure in a monoatomic gas increases linearly from 4 xx 10^5 N...
Text Solution
|
- The pressure in a monoatomic gas increases linearly from 4 xx 10^5 N...
Text Solution
|
- The pressure in a monoatomic gas increases linearly from 4 xx 10^5 N...
Text Solution
|
- The pressure in a monoatomic gas increases linearly from 4 xx 10^5 N...
Text Solution
|
- A monatomic ideal gas of two moles is taken through a cyclic process s...
Text Solution
|
- Figure depicts the working diagram (P-V graph) of a process correspond...
Text Solution
|
- Figure depicts the working diagram (P-V graph) of a process correspond...
Text Solution
|
- Figure depicts the working diagram (P-V graph) of a process correspond...
Text Solution
|
- Figure depicts the working diagram (P-V graph) of a process correspond...
Text Solution
|
- Figure shows the graph of pressure versus volume of an ideal gas, take...
Text Solution
|
- Figure shows the graph of pressure versus volume of an ideal gas, take...
Text Solution
|
- Figure shows the graph of pressure versus volume of an ideal gas, take...
Text Solution
|
- Figure shows the graph of pressure versus volume of an ideal gas, take...
Text Solution
|
- Draws the P-Tand V-T diagrams for an isobaric process of expansion, co...
Text Solution
|
- Find the efficiency of the thermo-dynamic cycle shown in figure for an...
Text Solution
|
- Figure shows a cyclic process ABCDBEA performed on an ideal cycle. If...
Text Solution
|
- Two moles of helium gas undergo cyclic processes shown as assuming the...
Text Solution
|