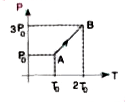
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMAL PROPERTIES OF MATTER
AAKASH SERIES|Exercise EXPANSION OF GASES (LECTURE SHEET) Straight Objective Type Questions (Boyle.s Law - applications)|5 VideosTHERMAL PROPERTIES OF MATTER
AAKASH SERIES|Exercise EXPANSION OF GASES (LECTURE SHEET) Straight Objective Type Questions (Kinetic theory of gases)|5 VideosTHERMAL PROPERTIES OF MATTER
AAKASH SERIES|Exercise EXPANSION OF LIQUIDS (PRACTICE SHEET) LEVEL-II (Integer/Subjective Type Questions)|4 VideosSYSTEM OF PARTICLES AND ROTATIONAL MOTION
AAKASH SERIES|Exercise PRACTICE EXERCISE|99 VideosTHERMODYNAMICS
AAKASH SERIES|Exercise EXERCISE - 3|33 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-THERMAL PROPERTIES OF MATTER-EXPANSION OF GASES (LECTURE SHEET) Straight Objective Type Questions (Ideal Gas equation - applications)
- For an ideal gas V-T curves as constant pressures P1 & P2 are shown in...
Text Solution
|
- Two different at constant. The relationship between volume V and the p...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- The pressure p of a gas is plotted against its absolute temperature T ...
Text Solution
|
- A non-linear triatomic gas is 50% dissociated into individual atoms. I...
Text Solution
|
- Pressure versus temperature graph of an ideal gas is as shown in figur...
Text Solution
|
- One mole of an ideal gas undergoes a process P= ( P0)/(1+ ((V0)/( V) )...
Text Solution
|
- A cylindrical tube of cross-sectional area A has two air tight frictio...
Text Solution
|
- Two cylinders fitted with pistons and placed as shown, connected with ...
Text Solution
|
- Two perfect gases at absolute temperatures T1 and T2 are mixed. There ...
Text Solution
|