Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL THERMODYANMICS
AAKASH SERIES|Exercise Exercise 3.1.1|8 VideosCHEMICAL THERMODYANMICS
AAKASH SERIES|Exercise Exercise 3.1.2|12 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|30 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise PRACTICE EXERCISE|50 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL THERMODYANMICS -Questions For Descriptive Answers
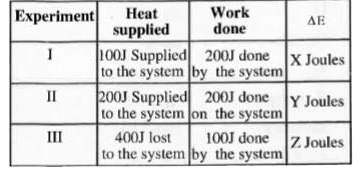
- From the observations given below, suggest the relation between X, Y a...
Text Solution
|
- How does heat of neutralisation value change with the chemical nature ...
Text Solution
|
- How does entropy changes with the transformation at different temperat...
Text Solution
|
- At 400K, 5 moles of an ideal gas expands isothermally and reversibly f...
Text Solution
|
- The molar heat capacity at constant volume of a system is 12.41J. mol^...
Text Solution
|
- Enthalpy of ammonia and water are -46.19KJ.mol^(-1) and -285.9KJ.mol^(...
Text Solution
|
- H(2)+Cl(2)to 2HCl,Delta H(1), ...
Text Solution
|
- The heat of combustion a sugar is -790KJmol^(-1). A person requires 23...
Text Solution
|
- Given that bond energies of N=N,H-H and N-H bonds as 945, 436 and 391 ...
Text Solution
|
- At constant pressure, the heat of combustion of carbon monoxide at 17^...
Text Solution
|
- What is the work done in a open vessel at 27^(@)C, when 92 grams of so...
Text Solution
|
- Certain amount of argon at 1 atm and 300 K expands reversibly and adia...
Text Solution
|
- In an insulated container, 1 mole of a liquid of molar volume 100 mL i...
Text Solution
|
- HCI is a strong acid, but HF is a weak acid. The enthalpy of neutralis...
Text Solution
|
- The enthalpy of vapourisation of water is 37.3 kl mol^(-1). Calculate...
Text Solution
|
- The standard enthalpy of hydrogenation of cyclohexene is119 kJmol^(-1)...
Text Solution
|
- Calculate the number of calories of energy released when 1 L of HCI is...
Text Solution
|
- Heats of atomisation of NH(3) and NH(2)H(4) are xKJmol^(-1)" and "yKJm...
Text Solution
|
- Standard enthalpies of C(6)H(6)(l),H(2)O(g) " and "CO(2)(g) are respec...
Text Solution
|
- Heat of formation of water and heats of combustion of ethylene and ace...
Text Solution
|
- At 427^@C, for a given change the values of DeltaG and DeltaH are -11,...
Text Solution
|