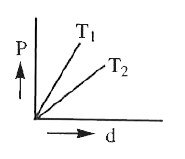
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In Boyles experiment for a given gas at different temperatures the gra...
Text Solution
|
- The graph drawn between pressure and volume lin Boyles law experiment ...
Text Solution
|
- In Boyles experiment for a given gas at different temperature the grap...
Text Solution
|
- Pressure versus temperature graph of an ideal gas is shown in figure. ...
Text Solution
|
- Pressure versus temperature graph of an ideal gas at constant volume V...
Text Solution
|
- बॉयल के अनुसार गैस के दाब व घनत्व में सम्बन्ध P = KD. सत्य है
Text Solution
|
- मोल आदर्श गैस के व्यवहार को एक वायुमण्डलीय दाब पर प्रदर्शित किया गया ह...
Text Solution
|
- Graphs between pressure and volume are drawn at different temperatures...
Text Solution
|
- Pressure versus temperature graph of an ideal gas is as shown in figur...
Text Solution
|