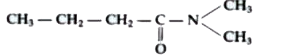Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AMINES
U-LIKE SERIES|Exercise SHORT ANSWER QUESTIONS (2 MARKS EACH)|41 VideosAMINES
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS-I (3 MARKS EACH)|27 VideosAMINES
U-LIKE SERIES|Exercise TRUE OR FALSE|6 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION B)|1 VideosBIOMOLECULES
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS - I|22 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-AMINES-VERY SHORT ANSWER QUESTIONS (1 MARK EACH)
- Write IUPAC name of the following compound : " "CH(3)NHCH(C...
Text Solution
|
- Write the IUPAC name of the given compound :
Text Solution
|
- Write the IUPAC name of the given compound :
Text Solution
|
- The conversion of primary aromatic amines into diazonium salts is know...
Text Solution
|
- Arrange the following in the decreasing order of their basic strength ...
Text Solution
|
- Rearrange the following in an increasing order of their basic strength...
Text Solution
|
- Direct nitration of aniline is not carried out. Explain why ?
Text Solution
|
- Give a chemical test to distinguish between ethylamine and aniline.
Text Solution
|
- Of methylamine and aniline which is a stronger base and why ?
Text Solution
|
- Why is an alkylamine more basic than ammonia ?
Text Solution
|
- Write the IUPAC name of the compound " "CH(2)(Cl)COCH(CH(3)...
Text Solution
|
- Rearrange the following compounds in an increasing order of their basi...
Text Solution
|
- Arrange the following compounds in an increasing order of basic streng...
Text Solution
|
- What is the role of HNO(3) in the nitrating mixture used for nitration...
Text Solution
|
- Why is NH(2) group of aniline acetylated before carrying out nitration...
Text Solution
|
- What is the product obtained when C(6)H(5)CH(2)NH(2) reacts with HNO(2...
Text Solution
|
- What is the best reagent to convert nitrile to primary amine ?
Text Solution
|
- What is Hinsberg's reagent ?
Text Solution
|
- Why is benzene diazonium chloride not stored and is used immediately a...
Text Solution
|
- Under what reaction conditions (acidic/basic), the coupling reaction o...
Text Solution
|