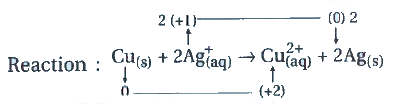Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-A|72 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-A (Try Yourself)|25 VideosCHEMISTRY IN EVERYDAY LIFE
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)|46 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ELECTROCHEMISTRY-SECTION-E MCQ.s Asked in GUJCET/Board Exam
- Calculate the equilibrium constant of the reaction: Cu((s))+2Ag((ag)...
Text Solution
|
- If for Ag|Ag((0.01M))^(+)||Ag((0.1M)^(+)|Ag, cell E(Ag^(+)|Ag)^(@)=0.8...
Text Solution
|
- If Fe|Fe^(2+)(xM)||Cu^(2+)(0.01M)|Cu has cell potential of 0.78V then ...
Text Solution
|
- At 25^(@)C temperature, if for given unknown half cell has 0.34 Volt p...
Text Solution
|
- Resistance of 1 N CH(3)COOH is 250 ohm. This conductive cell has const...
Text Solution
|
- Electrochemical cell Mg((S))|Mg((aq))^(2+)(xM)||Fe^(2+)(0.01M)|Fe((l...
Text Solution
|
- Which of the following relation is true for standard gibbs free energy...
Text Solution
|
- If 2.7 gm aluminum metal is deposited on electrodes when two different...
Text Solution
|
- If electrolysis of aqueous solution of CuSO(4) is carried out using gr...
Text Solution
|
- Which of the following reaction is true at 25^(@)C for given cell ? ...
Text Solution
|
- 22.2 gm Sn is deposited on electrode when 2 ampere current is pass thr...
Text Solution
|
- Which of the following reaction shows metal corrosion reaction in pres...
Text Solution
|
- Product of electrolytic cell does not depend on which of the followng ...
Text Solution
|
- Which of the following gives H(2) on cathode and O(2) on anode on elec...
Text Solution
|
- Which of the following statement is wrong with respect to metallic or ...
Text Solution
|
- Which of the following cell is concentration cell ?
Text Solution
|
- Resultant solution of electrolysis of concentrated NaCl solution is ....
Text Solution
|
- Metal A,B and C has reduction potentials, 0.34 volt, -0.80 volt and -0...
Text Solution
|
- Electrolytic cell containing molten nickel chloride and aluminium chlo...
Text Solution
|
- Oxidation potential of given hydrogen half cell at 25^(@)C is 0.118V t...
Text Solution
|
- Standard reduction potential of x,y and z are 0.75,-0.80 and -.25 volt...
Text Solution
|