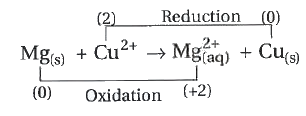A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-D NCERT Exemplar Solution (Short Answer Type Questions)|22 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-D NCERT Exemplar Solution (Match The Columns)|6 VideosELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-D NCERT Exemplar Solution (MCQ.s)|17 VideosCHEMISTRY IN EVERYDAY LIFE
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)|46 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ELECTROCHEMISTRY-SECTION-D NCERT Exemplar Solution (MCQ.s More Than One Options)
- The positive value of the standard electrode potential of Cu^(2+)//Cu ...
Text Solution
|
- E(cell)^(Theta) for some half-cell reactions are given below. On the b...
Text Solution
|
- E(cell)^(Theta)=1.1V for Daniel cell. Which of the following expressio...
Text Solution
|
- Conductivity of an electrolytic solution depends on . . . .
Text Solution
|
- Lamda(m)^(@)(H(2)O) is equal to
Text Solution
|
- What will happen during the electrolysis of aqueous solution of CuSO(4...
Text Solution
|
- What will happen during the electrolysis of aqueous solution of CuSO(4...
Text Solution
|
- Conductivity kappa, equal to
Text Solution
|
- Molar conductivity of ionic solution depends on
Text Solution
|
- For the given cel, Mg|Mg^(2+)||Cu^(2+)|Cu
Text Solution
|