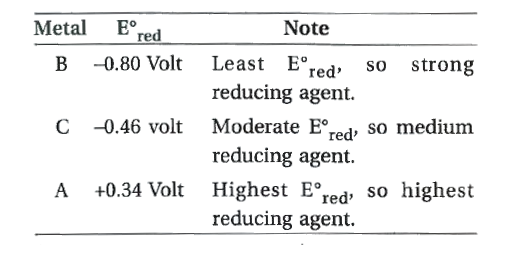A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION-E MCQ.s asked in JEE/NEET/AIEEE|63 VideosCHEMISTRY IN EVERYDAY LIFE
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJCET/BOARD EXAM)|37 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs ASKED IN GUJET/BOARD EXAMS)|46 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-ELECTROCHEMISTRY-SECTION-E MCQ.s Asked in GUJCET/Board Exam
- Which of the following cell is concentration cell ?
Text Solution
|
- Resultant solution of electrolysis of concentrated NaCl solution is ....
Text Solution
|
- Metal A,B and C has reduction potentials, 0.34 volt, -0.80 volt and -0...
Text Solution
|
- Electrolytic cell containing molten nickel chloride and aluminium chlo...
Text Solution
|
- Oxidation potential of given hydrogen half cell at 25^(@)C is 0.118V t...
Text Solution
|
- Standard reduction potential of x,y and z are 0.75,-0.80 and -.25 volt...
Text Solution
|
- What is the complete charging reaction of Ni-Cd storage cell ?
Text Solution
|
- How much moles of oxidizing agents are reduced by complete reaction of...
Text Solution
|
- Which of the following aqueous solution does not show straight line gr...
Text Solution
|
- Choose correct optio for working given cell : Pt|underset("1 bar")(C...
Text Solution
|
- During electrolysis of dilute aqueous CuSO(4) solution by inert electr...
Text Solution
|
- In which metal vessels aqueous CuSO(4) solution can be store ? E(Cu^...
Text Solution
|
- What is the oxidation potential of given hydrogen half cel at 1 bar pr...
Text Solution
|
- To obtain 5.85 gm nickel how long 10 ampere current should be passed t...
Text Solution
|
- What is the density of H(2)SO(4) solution when lead storage cell stop ...
Text Solution
|
- When value of equilibrium constant is more than 1, then for spontaneou...
Text Solution
|
- The electrochemical cell in which electrodes are same but their electr...
Text Solution
|
- Which of the following is responsible for transportation of negative i...
Text Solution
|
- Which products are obtained on anode and cathode respectively when ele...
Text Solution
|
- Molar conductivity of KCl, NaCl and KNO(3) are 150, 126 and 109 SC m^(...
Text Solution
|