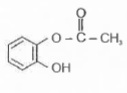
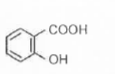
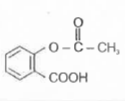
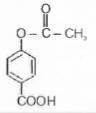
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-MOCK TEST 40-Example
- Product obtained by heating 4-Amino butanoic acid
Text Solution
|
- Bakelite is an example of
Text Solution
|
- Which among the following is a polyester?
Text Solution
|
- Ziegler-Natta catalyst is
Text Solution
|
- Monomer unit of Nylon 6 is
Text Solution
|
- Poly Beta-hydroxybutyrate-co-Beta-hydroxy valerate (PHBV) is obtained ...
Text Solution
|
- Incorrect statement among the following is
Text Solution
|
- The correct match is
Text Solution
|
- Drugs that blinfld to the receptor site and inhibit its natural functi...
Text Solution
|
- Cimetidine (Tegamet) and Ranitidine (Zantac) drugs are
Text Solution
|
- Among the following, indentify the pair of antihistamine drugs
Text Solution
|
- The class of chemical compounds used for the treatment of stress are c...
Text Solution
|
- Correct structure of Aspirin is
Text Solution
|
- Dettol, commonly used antiseptic is a mixture of
Text Solution
|
- Consider the following statements - (i) Antiseptics are chemical subs...
Text Solution
|
- Penicillin is an example of
Text Solution
|
- Drugs which produce insensibility to the vital functions bof nervous s...
Text Solution
|
- The incorrect statement with respect to saccharin is
Text Solution
|
- Identify the false characteristic regarding detergents
Text Solution
|
- Norethindrone is an example of synthetic progesterone derivative which...
Text Solution
|