Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
KUMAR PRAKASHAN|Exercise SECTION - C (MULTIPLE CHOICE QUESTION (MCQs))|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
KUMAR PRAKASHAN|Exercise SECTION - C (MCQs ASKED IN COMPETITIVE EXAM)|17 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
KUMAR PRAKASHAN|Exercise TRY YOUR SELF-4|1 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
KUMAR PRAKASHAN|Exercise Section -D SOLUTIONS OF NCERT EXEMPLAR PROBLEMS (Long Answer Type Questions )|7 Videos
KUMAR PRAKASHAN-CHEMICAL BONDING AND MOLECULAR STRUCTURE-SECTION - B (OBJECTIVE QUESTIONS)
- What is the similarity and difference in sigma and pi orbitals ?
Text Solution
|
- What is the difference in Mo energy level in Li(2) to N(2) and O(2) t...
Text Solution
|
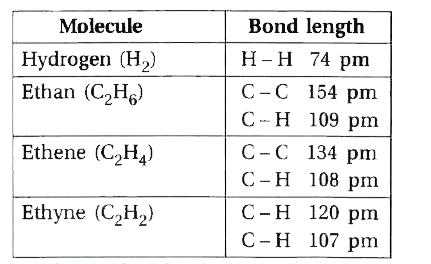
- Give bond length in Hydrogen, ethan, ethen, ethyne.
Text Solution
|
- Hydrogen bond is form in which molecules of the following ? Why ? CH...
Text Solution
|
- Give type of hydrogen bond in the following. Ice, water, liquid ammoni...
Text Solution
|
- State the relative stabiliry of N(2) , N(2)^(+), N(2)^(-) and N(2)^(2+...
Text Solution
|
- State tpe order of bond dissociation enthalpy in O(2), O(2)^(+), O(2)^...
Text Solution
|
- Is Neyon molecule Ne(2) possible ? Why ?
Text Solution
|
- Give bond order of H(2)^(+), He(2)^(+), He(2)^(2+).
Text Solution
|
- Give bond order of NO, NO^(+) , CN , CN^(-) and CO.
Text Solution
|
- Why the similar bond order in N(2) , NO^(+) , CN and CO ?
Text Solution
|
- Which bond angel is same in H(2)^(+) He(2)^(-) and He(2)^(2-)?
Text Solution
|
- Which are paramagnetic from O(2), O(2)^(-) and O(2)^(2) ?
Text Solution
|
- Which contain more strong H-bond in the followi!)g pair ? (i) H(2)O an...
Text Solution
|
- In O(2)^(-) and O(2)^(2-) which has more bond order ?
Text Solution
|
- Which of the following molecule have least bond order ? O(2) , O(2)^(+...
Text Solution
|
- When O(2)^(+) from O(2) and N(2)^(+) from N(2) are form then bond ord...
Text Solution
|
- State the requirements to form hydrogen bond.
Text Solution
|
- Sigma bond is not f orme in which overlapping of the following ? (i)...
Text Solution
|
- Which bond angle is high from PH(3) and PH(4)^(+) ? Why ?
Text Solution
|