Similar Questions
Explore conceptually related problems
Recommended Questions
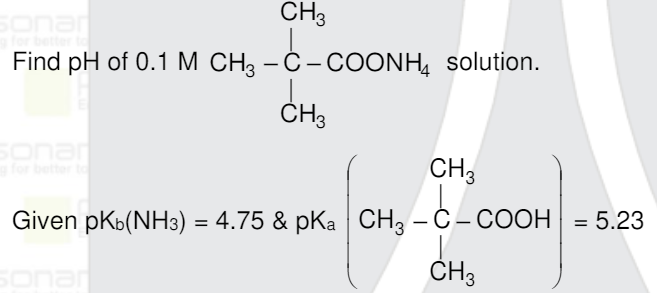
- Find pH of 0.1M
Text Solution
|
- 25.0 mL of 0.1M NaOh is titred with 0.1M HC1. Calculate pH when: i. ...
Text Solution
|
- The pH of 0.1M solution of the following salts decreases in the order
Text Solution
|
- Calcualte the pH at the equivalence point when a solution of 0.1M acet...
Text Solution
|
- The pH of a solution obtained by mixing 100mL of 0.1M HCI and 9.9mL of...
Text Solution
|
- pH of 0.1M Acetic acid is
Text Solution
|
- यदि साइनिक अम्ल (HCNO ) के 0.1M विलयन की pH , 2 .34 हो तो अम्ल ...
Text Solution
|
- What is the pH of 0.1M HCL solution?
Text Solution
|
- The ionization constant of chloroacetic acid is 1.65xx10^(-3). What wi...
Text Solution
|