Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 1)
SUNSTAR PUBLICATION|Exercise PART - D|25 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 1)
SUNSTAR PUBLICATION|Exercise PART - B|12 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|26 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 2)
SUNSTAR PUBLICATION|Exercise PART - D|28 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 1)-PART - C
- Write the equations for the reactions involved in the leaching of alum...
Text Solution
|
- In the manufacture of ammonia by Haber's process: i) Mention the opt...
Text Solution
|
- a) Complete the following equations: i) PbS((s))+4O(3(g))rarr ii) ...
Text Solution
|
- Write the structure of oleum (H(2)S(2)O(7))
Text Solution
|
- a) Give reason: i) Fluorine exhibits only one oxidation state wherea...
Text Solution
|
- b) Write the missing product: NH(3)+3Cl(2"(excess)")rarr?+3HCl.
Text Solution
|
- Explain the preparation of potassium permanganate from MnO(2) Write th...
Text Solution
|
- Give reasons: i) Generally there is increase in density along 3d ser...
Text Solution
|
- Give reasons: Third ionisation enthalpy of manganese is unusually h...
Text Solution
|
- Which of the following ions is coloured iņ aqueous solution? i) Sc^(...
Text Solution
|
- Using valence bond theory account for the geometry and magnetic nature...
Text Solution
|
- a) In the complex compound represented by CoCl(3).4NH(3), how many amm...
Text Solution
|
- b) What type of structural, isomerism is exhibited by the complex Co (...
Text Solution
|
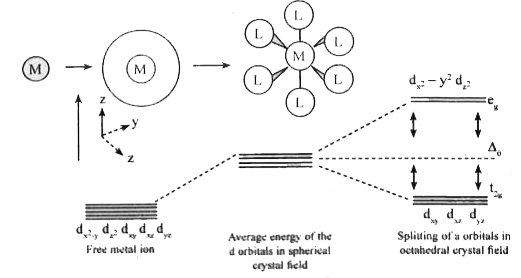
- c) Between t(2g) and e(g) sets of d-orbitals of a central metal in an ...
Text Solution
|