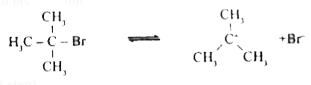Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 1)
SUNSTAR PUBLICATION|Exercise PART - C|14 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|26 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 2)
SUNSTAR PUBLICATION|Exercise PART - D|28 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 1)-PART - D
- What is the composition of the cathode in the lead storage battery?
Text Solution
|
- Name the product discharged at the anode during the electrolysis of an...
Text Solution
|
- Derive an integrated rate equation for the rate constant of a first-or...
Text Solution
|
- b) In the equation, rate =Z(AB)xxe^((E(a))/(R), what does the term e^(...
Text Solution
|
- What is the effect of a catalyst on DeltaG of a reaction?
Text Solution
|
- i) What type of adsorption involves Van der Waals force of attraction?...
Text Solution
|
- i) What is peptization? ii) What is the dispersed phase in a gel? ...
Text Solution
|
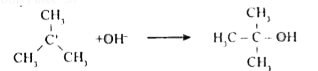
- Discuss the mechanism of hydrolysis of tert - butyl bromide.
Text Solution
|
- Identify the missing reactant/product in each of the following: i) 2...
Text Solution
|
- c) Between which is an allylic chloride?
Text Solution
|
- Write the equations involved in the preparation of phenol from cumene.
Text Solution
|
- i) Give the IUPAC name of the product formed when tertiary butyl alcoh...
Text Solution
|
- Explain Stephen's reduction with an example.
Text Solution
|
- Complete the following equations: i) 2HCHO+" conc. KOH"rarr ii) CH...
Text Solution
|
- Explain Hoffmann bromamide degradation reaction and write the general ...
Text Solution
|
- i) Give reason: Aniline is a weaker base than ammonia. ii) C(6)H(5)N...
Text Solution
|
- Write the Haworth structure of D - sucrose. Why is a non - reducing su...
Text Solution
|
- i) How many peptide bonds are in a hexapeptide? ii) Write the genera...
Text Solution
|
- i) Name the polymer whose partial structure is represented by ii) Wh...
Text Solution
|
- i) Name the catalyst used in the manufacture of high density polythene...
Text Solution
|