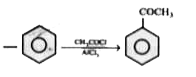
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH SERIES|Exercise LECTURE SHEET ( EXERCISE-V (AROMATICITY AND ACID-BASE PROPERTIES , AROMATIC ELECTROPHILLIC SUBSTITUTION(MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS )))|12 VideosHYDROCARBONS
AAKASH SERIES|Exercise LECTURE SHEET ( EXERCISE-V (AROMATICITY AND ACID-BASE PROPERTIES , AROMATIC ELECTROPHILLIC SUBSTITUTION(LINKED COMPREHENSION TYPE QUESTIONS(PASSAGE-I))))|5 VideosHYDROCARBONS
AAKASH SERIES|Exercise LECTURE SHEET ( EXERCISE-IV (DIENES (INTEGER TYPE QUESTIONS)))|3 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|35 VideosHYDROGEN AND ITS COMPOUNDS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|20 Videos
AAKASH SERIES-HYDROCARBONS-LECTURE SHEET ( EXERCISE-V (AROMATICITY AND ACID-BASE PROPERTIES , AROMATIC ELECTROPHILLIC SUBSTITUTION( STRAIGHT OBJECTIVE TYPE QUESTION )))
- Which of the following reactions, aromatic characters retained ?
Text Solution
|
- Among the following compounds, the decreasing order of reactivity towa...
Text Solution
|
- Which of the following is not aromatic ?
Text Solution
|
- . What is 'x'
Text Solution
|
- Which of the following is least reactive towards electrophillic additi...
Text Solution
|
- Which order are correct regarding B.P
Text Solution
|
- The number of sigma and pi (TT) bonds present in benzene respectively ...
Text Solution
|
- Some meta directing substituents in aromatic substitution are given wh...
Text Solution
|
- The non-aromatic compound among the following is
Text Solution
|
- Aromatic compounds containing benzene are termed as
Text Solution
|
- The presence of delocalized pi - electrons in benzene indicates that i...
Text Solution
|
- The C-C bond in benzene can be determined by
Text Solution
|
- <img src="https://d10lpgp6xz60nq.cloudfront.net/physicsimages/AKSTRGAO...
Text Solution
|