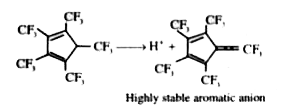
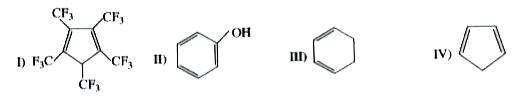A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - V (LEVEL-II (STRAIGHT OBJECTIVE TYPE QUESTIONS )))|14 VideosHYDROCARBONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - V (LEVEL-II (MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS )))|11 VideosHYDROCARBONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - IV (LEVEL-II (MATRIX MATCHING TYPE QUESTIONS )))|2 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|35 VideosHYDROGEN AND ITS COMPOUNDS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-HYDROCARBONS-PRACTICE SHEET (EXERCISE - V (LEVEL-I (STRAIGHT OBJECTIVE TYPE QUESTIONS )))
- Aromatic compounds containing benzene are termed as
Text Solution
|
- The presence of delocalized pi - electrons in benzene indicates that i...
Text Solution
|
- The C-C bond in benzene can be determined by
Text Solution
|
- Complete the following reaction
Text Solution
|
- . Here , A refers to
Text Solution
|
- In the compound C6H5Z which of the following is predominantly ortho/pa...
Text Solution
|
- Rank the following compounds in order of decreasing reactivity towards...
Text Solution
|
- The relative rates of mononitration of C6H5 - Z , " where " Z = CH3 - ...
Text Solution
|
- Which of the following compound/s are carcinogenic?
Text Solution
|
- Arrange the following compounds in increasing order of polarity
Text Solution
|
- Enthalpy of hydrogenation of cyclohexene is -119Kj/mol and that of ben...
Text Solution
|
- Select the species which is not aromatic.
Text Solution
|
- What makes the following compound aromatic?
Text Solution
|
- Arrange the following compounds in increasing order of their basic str...
Text Solution
|
- What is the correct order of increasing acidic strength of the followi...
Text Solution
|
- In benzene molecule there are three pi bonds and
Text Solution
|
- Which of the following reaction takes place when a mixture of concentr...
Text Solution
|
- Resonance energy of benzene is
Text Solution
|
- Which group is meta directing?
Text Solution
|