Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROCARBONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - VI (LEVEL-I (STRAIGHT OBJECTIVE TYPE QUESTIONS )))|22 VideosHYDROCARBONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - VI (LEVEL-II (STRAIGHT OBJECTIVE TYPE QUESTIONS )))|12 VideosHYDROCARBONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - V (LEVEL-II (LINKED COMPREHENSION TYPE QUESTIONS (PASSAGE-I))))|3 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|35 VideosHYDROGEN AND ITS COMPOUNDS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-HYDROCARBONS-PRACTICE SHEET (EXERCISE - V (LEVEL-II (INTEGER TYPE QUESTIONS)))
- How many of the following undergo electrophilic nitration more rapidly...
Text Solution
|
- The number of p atomic orbitals involved in the formation of a benzene...
Text Solution
|
- The substituents which are deactivating but ortho para directing in ar...
Text Solution
|
- The number of compounds which are more reactive towards electrophilic ...
Text Solution
|
- The number compounds which undergo nucleophilic substitution below 50^...
Text Solution
|
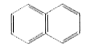
- An organic compound with the formula C(10)H(8) absorbs 5H2 during hydr...
Text Solution
|
- How many of the following compounds undergo electrophilic substitution...
Text Solution
|
- The total no. of pi electrons present in the benzene are
Text Solution
|
- From the list below, how many of them are aromatic?
Text Solution
|
- How many isomers of C7H8 O exists that has phenyl ring ?
Text Solution
|
- If the following compound is treated with Br2 - Fe, how many mono brom...
Text Solution
|