A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE SHEET ( LEVEL-II (LECTURE SHEET (ADVANCED) (MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)))|20 VideosHYDROCARBONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE SHEET ( LEVEL-II (LECTURE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS(PASSAGE-I))))|3 VideosHYDROCARBONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - VI (LEVEL-II (MATRIX MATCHING TYPE QUESTIONS )))|4 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|35 VideosHYDROGEN AND ITS COMPOUNDS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|20 Videos
AAKASH SERIES-HYDROCARBONS-ADDITIONAL PRACTICE SHEET ( LEVEL-I (LECTURE SHEET (MAIN) (STRAIGHT OBJECTIVE TYPE QUESTIONS)))
- . Which one of the following is major product ?
Text Solution
|
- , unknown A is
Text Solution
|
- Van't-Haff factor (i) for this reaction is
Text Solution
|
- The presence of which one of the following groups on benzene nucleus a...
Text Solution
|
- Which species will undergo electrophilic substitution reaction most ea...
Text Solution
|
- Which of the following species is expected to have maximum enthalpy in...
Text Solution
|
- Which of the following aromatic compound will undergo nitration most e...
Text Solution
|
- Which of the following is meta directing group?
Text Solution
|
- Identify the correct order of reactivity in electrophilic substitution...
Text Solution
|
- Which one of the following will undergo meta substitution on mono chlo...
Text Solution
|
- The highest yield of m product is possible by the electrophilic substi...
Text Solution
|
- Which of the following is not an aromatic compound:
Text Solution
|
- Which of the following is most reactive towards sulphonation?
Text Solution
|
- The conversion can be effected using
Text Solution
|
- In the presence of iron catalyst, benzene reacts with chlorine to form
Text Solution
|
- . Where B is major product B is
Text Solution
|
- Product. Major product in above reaction is
Text Solution
|
- How many distinct alkene products are possible when alkyl lodide below...
Text Solution
|
Text Solution
|
- The general formula of Alkene's is same as
Text Solution
|
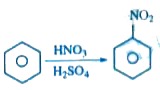 Van't-Haff factor (i) for this reaction is
Van't-Haff factor (i) for this reaction is