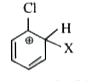
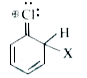
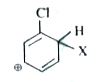A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE SHEET ( LEVEL-II (LECTURE SHEET (ADVANCED) (MORE THAN ONE CORRECT ANSWER TYPE QUESTIONS)))|20 VideosHYDROCARBONS
AAKASH SERIES|Exercise ADDITIONAL PRACTICE SHEET ( LEVEL-II (LECTURE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS(PASSAGE-I))))|3 VideosHYDROCARBONS
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - VI (LEVEL-II (MATRIX MATCHING TYPE QUESTIONS )))|4 VideosGENERAL ORGANIC CHEMISTRY
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|35 VideosHYDROGEN AND ITS COMPOUNDS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|20 Videos
AAKASH SERIES-HYDROCARBONS-ADDITIONAL PRACTICE SHEET ( LEVEL-I (LECTURE SHEET (MAIN) (STRAIGHT OBJECTIVE TYPE QUESTIONS)))
- ter-Butyl benzene can be obtained by
Text Solution
|
- Which of the following statement is true.
Text Solution
|
- Which of the following structure is least stable?
Text Solution
|
- Which of the following is comparatively stable?
Text Solution
|
- Which of the following is not an example of friedel-craft reaction.
Text Solution
|
- When carbon tetrachloride is treated with excess of Benzene in presenc...
Text Solution
|
- Which of the following statement is true?
Text Solution
|
- What happens when aniline is treated with methyl chloride in presence ...
Text Solution
|
- Which of the following is a nonaromatic.
Text Solution
|
- Which of the following structures are aromatic
Text Solution
|
- Purine has four nitrogen atoms, which one of them is expected to be ba...
Text Solution
|
- C6H6 +CH2Cl2 overset(AlCl3)to C , excess .
Text Solution
|
- The correct order of increasing basic nature for the following compoun...
Text Solution
|
- The increasing order of the acidic nature of the following compounds i...
Text Solution
|
- In Huckel's (4n+2) rule of for aromaticity, 'n' represents
Text Solution
|
- Which of the following are conjugated dienes?
Text Solution
|
- What is the correct order of exothermicity for hydrogenation of the fo...
Text Solution
|
- Which of the following arrangements of p orbitals gives the lowest ene...
Text Solution
|
- Which of the following dienes undergoes the least exothermic hydrogena...
Text Solution
|
- Which of the following is not formed by the addition of 1 mole of HCl ...
Text Solution
|