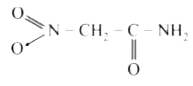A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following molecule have least oxidation state of the nitr...
Text Solution
|
- Which of the following oxides of nitrogen is a linear and asymmertrica...
Text Solution
|
- In which pair of substances do the nitrogen atoms have the same oxidat...
Text Solution
|
- Which of the following has least oxidation state of Fe?
Text Solution
|
- In which of the following compounds the oxidation state of nitrogen is...
Text Solution
|
- A gaseous nitrogen oxide contains 30.4% of nitrogen, one molecule of w...
Text Solution
|
- In which of the following compounds , the nitrogen atom exhibits negat...
Text Solution
|
- निम्न में से किस यौगिक में नाइट्रोजन की ऑक्सीकरण अवस्था, असत्य है
Text Solution
|
- Oxygen exhibits least oxidation state in
Text Solution
|