A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
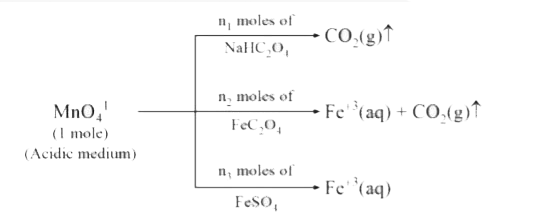
- In the above set of redox reactions, where MnO(4)^(-1) is the only ox...
Text Solution
|
- A reducing agent in a redox reaction undergoes
Text Solution
|
- Name the substance oxidised, reduced, oxidising agent and reducing age...
Text Solution
|
- नाइट्रिक अम्ल केवल ऑक्सीकारक के रूप में कार्य करता है जबकि नाइट्रस अम्...
Text Solution
|
- Oxidising and redeecing Agents| Tyoes of Redox Reactions
Text Solution
|
- Identify the substances oxidised, substance reduced, oxidising agent a...
Text Solution
|
- Types of Redox Reaction |Oxidising Agent And Reducing Agent
Text Solution
|
- Is Pb^(4+) a reducing agent or an oxidising agent? Why?
Text Solution
|
- What is correct for redox reaction ? (i) Reducing agent undergoes ox...
Text Solution
|