A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JMD PUBLICATION-CHEMICAL KINEMATICS-EXAMPLE
- The role of a catalyst in a chemical reaction is to change:
Text Solution
|
- The units of rate constant for first order equation.
Text Solution
|
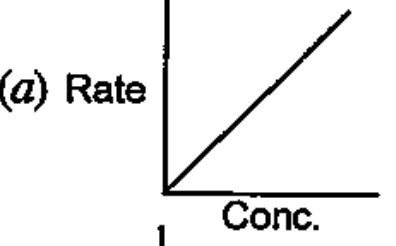
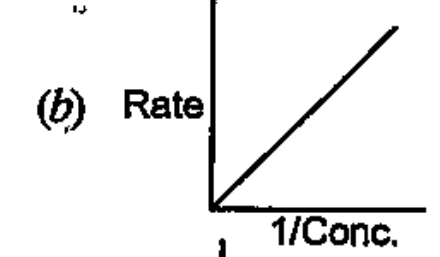
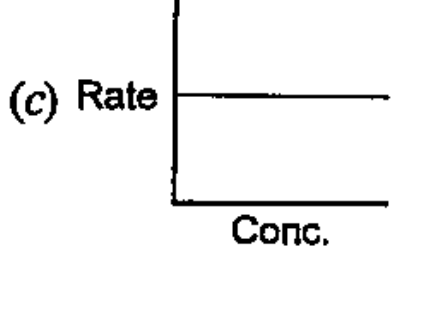
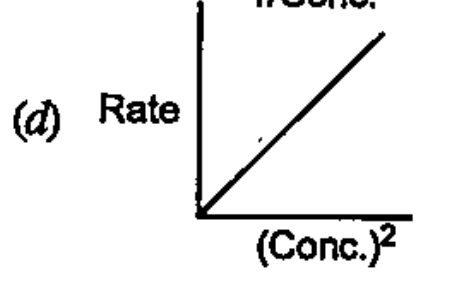
- Which of the following graphs corresponds to first order reaction:
Text Solution
|
- Rate constant of a reaction depends upon:
Text Solution
|
- Arrhenius equation is
Text Solution
|
- The chemical reactions in which the reactants require high amount of a...
Text Solution
|
- Which of the following does not influence the reaction rate?
Text Solution
|
- A reaction was found to be of second order with respect to concentrati...
Text Solution
|
- The value of k for a reaction is 2.96xx10^(30)s^-1 , what is the order...
Text Solution
|
- The units of rate constant for first order equation.
Text Solution
|
- The rate constant of a reaction has same units as the rate of reaction...
Text Solution
|
- The rate constant of a reaction has s^-1 units. The reaction is of …..
Text Solution
|
- The rate constant of reaction is 3xx10^-3 atm^-2sec^-1 . The order of...
Text Solution
|
- The order of a single step reaction can be
Text Solution
|
- The molecularity of a reaction can never be a fraction.
Text Solution
|
- Unit of rate constant for a second order reaction are L mol^-1 s^-1.
Text Solution
|
- The molecularity of a reaction can never be a fraction.
Text Solution
|
- Order of a reaction can be zero.
Text Solution
|
- Molecularity of a reaction cannot be zero.
Text Solution
|
- The half life period of a zero order reaction is independent of initia...
Text Solution
|