Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
JMD PUBLICATION-CHEMICAL KINEMATICS-EXAMPLE
- The decomposition of N2 O5 in carbon tetrachloride solution has been ...
Text Solution
|
- Reaction between NO2 and F2 to give NO2F takes place by the following...
Text Solution
|
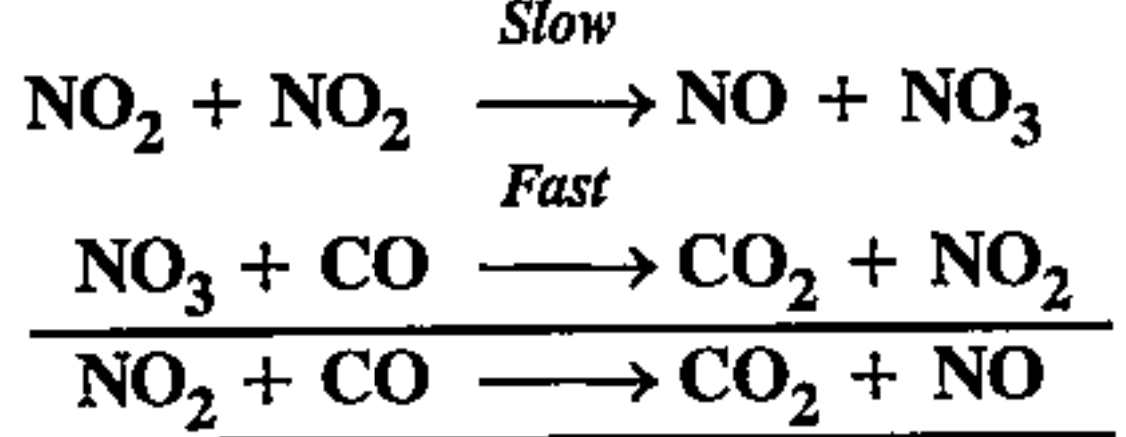
- Reaction between NO2 and CO to give CO2 and NO takes place by the fol...
Text Solution
|
- Thermal decomposition of dinitrogen penta oxide takes place by the fol...
Text Solution
|
- The half-life for radioactive decay of .^14C is 5730 years. An archaeo...
Text Solution
|
- A first order reaction has a rate constant 1.15 xx 10^-3 s^-1. How lo...
Text Solution
|
- Time required to decompose SO2 Cl2 to half of its initial amount Is 60...
Text Solution
|
- Show that the time required for 99% completion of a first order reacti...
Text Solution
|
- A first order reaction takes 40 min for 30% completion. Calculate t(1/...
Text Solution
|
- A first order reaction is 20% complete in the 10 minutes. Calculate th...
Text Solution
|
- The rate constant for a first order reaction is 80 s^-1. How much time...
Text Solution
|
- The rate constant for a first order reaction Is 90 s^-1 .How much time...
Text Solution
|
- First order reaction is found to have rate constant, k= 5.5xx 10^(-14)...
Text Solution
|
- Calculate two third life of a first order reaction having k=5.48xx 10^...
Text Solution
|
- Find the half life period of first order reaction whose rate constant,...
Text Solution
|
- The half life period for a reaction of first order is 2.31xx10^3 min. ...
Text Solution
|
- The rate constant for a first order reaction is 3.0 xx 10^-4 min^-1. H...
Text Solution
|
- Calculate the time required for the completion of 90% of a reaction of...
Text Solution
|
- The decomposition of A into product has value of k as 4.5 xx 10^3 s^-1...
Text Solution
|
- The rate of the chemical reaction doubles for an increase of 10 K In a...
Text Solution
|