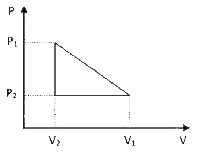
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
QUESTION PAPER 2014
WB JEE PREVIOUS YEAR PAPER|Exercise PHYSICS (CATEGORY II)|10 VideosQUESTION PAPER 2014
WB JEE PREVIOUS YEAR PAPER|Exercise PHYSICS (CATEGORY III)|3 VideosQUESTION PAPER 2013
WB JEE PREVIOUS YEAR PAPER|Exercise Category-III|5 VideosQUESTION PAPER 2015
WB JEE PREVIOUS YEAR PAPER|Exercise PHYSICS (CATEGORY III)|5 Videos
Similar Questions
Explore conceptually related problems
WB JEE PREVIOUS YEAR PAPER-QUESTION PAPER 2014-PHYSICS (CATEGORY III)
- One mole of a van der Waals' gas obeying the equation (P + a/(V^2)) ...
Text Solution
|
- Half of the space between the plates of a parallel-plate capacitor is ...
Text Solution
|
- A heating element of resistance r is fitted inside an adiabatic cylind...
Text Solution
|
- A stream of electrons and protons are directed towards a narrow slit i...
Text Solution
|