A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
WB JEE PREVIOUS YEAR PAPER-QUESTION PAPER 2016-CHEMISTRY
- Which of the following is the correct option for free expansion of an ...
Text Solution
|
- Assign the Bravais lattice type of the following unit cell structure.
Text Solution
|
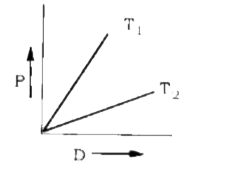
- Pressure (P) vs. density (D) curve for an ideal gas at two different t...
Text Solution
|
- Which of the following compounds is least effective in precipitating F...
Text Solution
|
- 75% of a first order reaction was completed in 32 min. When would 50% ...
Text Solution
|
- Which one of the following does not produce O2 as the only gaseous pr...
Text Solution
|
- Which of the following is true in respect of adsorption ?
Text Solution
|
- Which property that polyacetylene exhibits is unusual for an organic ...
Text Solution
|
- Which statement is incorrect?
Text Solution
|
- In the alumino -thermite process, aluminium acts as
Text Solution
|
- Consider the following reaction: 6NaOH + 3Cl(2) rarr5NaCl+A + 3H(2)O
Text Solution
|
- A sudden large difference between the values of second and third ioniz...
Text Solution
|
- Na(2)O2 is produced in reaction between H2O2 and NaOH. Here the role...
Text Solution
|
- Which statement is incorrect about complexes formed by the lanthanoids...
Text Solution
|
- m - dinitrobenzene can be converted to m - nitroaniline by reduction w...
Text Solution
|
- The correct IUPAC name of H(3) C - C (CH(3))(2) -CH = CH2 is
Text Solution
|
- Among the following compounds , the one which would not form a white p...
Text Solution
|
- Which combination of reagents used in the indicated order will give m ...
Text Solution
|
- Which of the statements (A) - (D) about the reaction profile below is ...
Text Solution
|
- Which of the following is the major product when one mole of propanone...
Text Solution
|