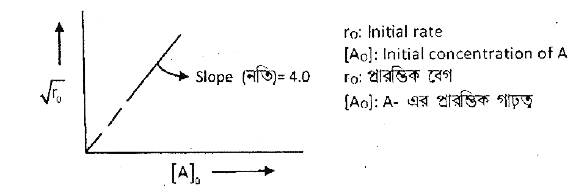A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
QUESTION PAPER 2019
WB JEE PREVIOUS YEAR PAPER|Exercise CHEMISTRY (CATEGORY -III)|5 VideosQUESTION PAPER 2019
WB JEE PREVIOUS YEAR PAPER|Exercise CHEMISTRY (CATEGORY -III)|5 VideosQUESTION PAPER 2018
WB JEE PREVIOUS YEAR PAPER|Exercise CHEMISTRY|40 VideosQUESTION PAPER 2021
WB JEE PREVIOUS YEAR PAPER|Exercise CHEMISTRY (CATEGORY -I)|40 Videos
Similar Questions
Explore conceptually related problems
WB JEE PREVIOUS YEAR PAPER-QUESTION PAPER 2019-CHEMISTRY (CATEGORY -II)
- At constant pressure, the heat of formation of a compound is not depen...
Text Solution
|
- A copper coin was electroplated with Zn and then heated at high temper...
Text Solution
|
- Oxidation of allyl alcohol with a peracid gives a compound of molecula...
Text Solution
|
- The total number of isomeric linear dipeptides which can be synthesize...
Text Solution
|
- The kinetic study of a reaction like vA to P at 300 K provides the fo...
Text Solution
|