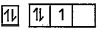
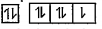
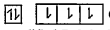A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-STRUCTURE OF ATOM-QUESTION TYPE
- What is the order of increasing bond angle of the following ? What is ...
Text Solution
|
- The atomic orbitals are progressively filled in order of increasing en...
Text Solution
|
- The orbital diagram in which both Pauli's exclusion principle and Hund...
Text Solution
|
- Who modified Bohr's theory by introducing elliptical orbits for electr...
Text Solution
|
- The following quantum numbers are possible for how many orbitals ? n...
Text Solution
|
- The 19th electron of chromium has which of the following sets of quant...
Text Solution
|
- Which of the following sets of quantum numbers is not possible ?
Text Solution
|
- Which one of the following pairs of ions have the same electronic conf...
Text Solution
|
- In a hydrogen atom if the energy of an electron in the ground state is...
Text Solution
|
- Which of the following ions has the maximum magnetic moment ?
Text Solution
|
- In hydrogen atom, energy of first excited state is -3.4 eV. Then find ...
Text Solution
|
- The wavelength associated with a golf ball weighing 200g and moving at...
Text Solution
|
- The quantum numbers +1/2 and -1/2 for the electron spin represent :
Text Solution
|
- Identify the least stable among the following
Text Solution
|
- If the nitrogen atom had electronic configuration is 1s^7, it would ha...
Text Solution
|
- List the quantum numbers (n and l) of electrons for 3d-orbital.
Text Solution
|
- Which quantum number specifies the shape of an orbital in an atom ?
Text Solution
|
- What is the value of e/m for an electron ?
Text Solution
|
- How many unpaired electrons are there in Ni^(2+) ion ?
Text Solution
|
- Differentiate between proton and photon.
Text Solution
|