Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- What is the bond angle and shape of CO2 ?
Text Solution
|
- Why H2O molecules remain associated in the liquid form ?
Text Solution
|
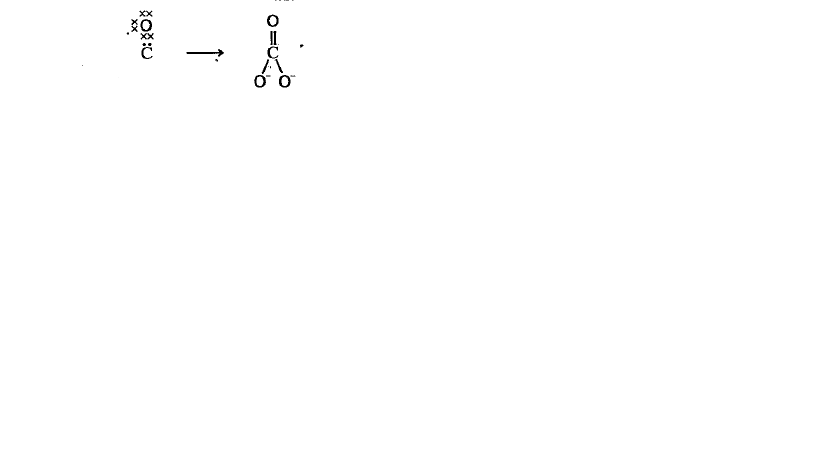
- Write the electronic structure of CO3^(-2) and NH4^+ ions with lines a...
Text Solution
|
- What type of hybridisation would account in the following cases ? BF3...
Text Solution
|
- What type of hybridisation would account in the following cases ? BeC...
Text Solution
|
- AB, CD, EF are three compounds having electronegativity difference bet...
Text Solution
|
- Write down the molecular orbital configuration of NO^+ and NO^- .
Text Solution
|
- Explain why HF is more polar than HCl .
Text Solution
|
- What is the state of hybridisation of central atom in the following mo...
Text Solution
|
- The hydrogen bonding in ammonia is less pronounced than in water. Why ...
Text Solution
|
- Distinguish between bonding and antibonding orbitals.
Text Solution
|
- Write the molecular orbital configuration of O2 .
Text Solution
|
- The compound in which carbon atom uses only its sp^ 3 - hybrid orbital...
Text Solution
|
- The paramagnetic behaviour of O2 molecules is best explained by :
Text Solution
|
- The molecule which has zero dipole moment is :
Text Solution
|
- A covalent bond is formed between the atoms by the overlapping of orbi...
Text Solution
|
- Length of hydrogen bond ranges from 2.5 overset@A to :
Text Solution
|
- A regular hexagonal crystalline lattice of ice is mostly formed by :
Text Solution
|
- In which of the following gaseous molecules, the ionic character of th...
Text Solution
|
- sp^3-hybridisation is important in describing the bonding in :
Text Solution
|