Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-B OBJECTIVE QUESTIONS (SHORT QUESTIONS)|56 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-B OBJECTIVE QUESTIONS (TRUE/FALSE)|6 VideosSTRUCTURE OF ATOM
KUMAR PRAKASHAN|Exercise QUESTINS PAPER FROM MODULE (SECTION -D)|2 VideosTHE s-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise Section-D NCERT Exemplar Solution (Long Answer Type)|8 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-THE P-BLOCK ELEMENTS-SECTION-D NCERT EXEMPLAR SOLUTION (MCQs) LONG ANSWER
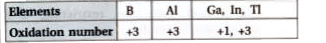
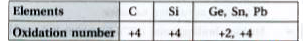
- Discuss the pattern of variation in the oxidation states of (i) B to ...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Describe the general trends in the following properties of the element...
Text Solution
|
- Account for the following observations : AlCl3 is a Lewis acid.
Text Solution
|
- Account for the following observations : Though fluorine is more ele...
Text Solution
|
- Account for the following observations : PbO2 is a stronger oxidizi...
Text Solution
|
- Account for the following observations : The +1 oxidation state of t...
Text Solution
|
- When aqueous solution of borax is acidified with hydrochloric acid, a ...
Text Solution
|
- Three pairs of compounds are given below. Identify that compound in ea...
Text Solution
|
- Three pairs of compounds are given below. Identify that compound in ea...
Text Solution
|
- Three pairs of compounds are given below. Identify that compound in ea...
Text Solution
|
- BCl3 exists as monomer whereas AlCl3 is dimerized through halogen brid...
Text Solution
|
- Boron fluoride exists as BF3 but boron hydride doesn't exist as BF3. G...
Text Solution
|