Similar Questions
Explore conceptually related problems
MBD-PERIODIC CLASSIFICATION OF ELKHOENTS-EXAMPLE
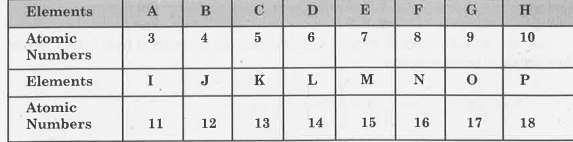
- The following tables shows the position of six elements A, B, C, D, E ...
Text Solution
|
- The following tables shows the position of six elements A, B, C, D, E ...
Text Solution
|
- The question refers to the elements of the periotic table with atomic ...
Text Solution
|
- An element belongs to 3rd period and 17th group of periodic table.Find...
Text Solution
|
- How does atomic size of elements vary on moving from left to right in ...
Text Solution
|
- How does atomic size of elements vary on moving from top to bottom in...
Text Solution
|
- Define periodic law. Why was it necessary to change the basis of calss...
Text Solution
|
- What do you understand by the term periodicity? Do the properties of t...
Text Solution
|
- What are limitation of doberiner's classification of elements?
Text Solution
|
- What property do all elements in the same column of the periodic table...
Text Solution
|
- Indicate the atomic number of non metals of period 3 of modern period...
Text Solution
|
- Indicate the atomic number of elements of period 3 of modern periodic ...
Text Solution
|
- Indicate the atomic number of elements of period 3 of modern periodic ...
Text Solution
|
- Indicate the atomic number of elements of period 3 of modern periodic ...
Text Solution
|
- Define atomic radius, give its units.
Text Solution
|
- How does atomic radius vary down a group and along a period?
Text Solution
|
- Write down the electronic configuration of elements with atomic number...
Text Solution
|
- Locate the following group in the table:alkali metals
Text Solution
|
- Locate the following group in the table: halogens
Text Solution
|
- Locate the following group in the table: alkaline earth metals
Text Solution
|