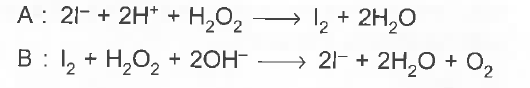A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-TEST 5-EXAMPLE
- Choose the incorrect statement.
Text Solution
|
- Group(s) of periodic table not forming hydrides is/are -
Text Solution
|
- Consider the reaction H2O2
Text Solution
|
- Match the Column-I to Column-II
Text Solution
|
- Equivalent mass of KMnO4 in acidic and basic medium respectively [M = ...
Text Solution
|
- Identify the compound in which S has highest oxidation state.
Text Solution
|
- Given : Identify the cell which will give maximum EMF^o
Text Solution
|
- What is the average oxidation state of oxygen In S2O8^2-
Text Solution
|
- Identity the disproportionaüon reaction.
Text Solution
|
- Which of the following arrangement represents increasing oxidation num...
Text Solution
|
- In which of the following compounds, an element exhibits two different...
Text Solution
|
- Consider the following reactions. I. 2PbO + 4HCl to 2PbCl2 + 2H2O ll....
Text Solution
|
- The most basic oxide among the following is -
Text Solution
|
- Among the following the least soluble sulphate is
Text Solution
|
- Thermal stability of hydrides of alkali metals -
Text Solution
|
- During manufacturing of sodium hydroxide castner kellner cell is -
Text Solution
|
- The abnormally smaller size of Ga among its group can be explained by ...
Text Solution
|
- The oxide which is amphoteric in nature is -
Text Solution
|
- When alkali metal is dissolved in liquid NH3 the blue colour of the di...
Text Solution
|
- Beryllium hydride forms polymeric structure in solid state due to exis...
Text Solution
|