Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CHETANA PUBLICATION-Periodic Classifications of ELements-EXAMPLE
- In the following table, seven elements P,Q,R,S,T,U and V (here letters...
Text Solution
|
- In the following table, seven elements P,Q,R,S,T,U and V (here letters...
Text Solution
|
- In the following table, seven elements P,Q,R,S,T,U and V (here letters...
Text Solution
|
- Write the answers of the question with refernce to the structure of th...
Text Solution
|
- Write the answers of the question with reference to the structure of t...
Text Solution
|
- Write the answers of the question with refernce to the structure of th...
Text Solution
|
- Draw the electronic configuration of the period 2 element of first gro...
Text Solution
|
- A part of periodic table is shown in the following figure Write the...
Text Solution
|
- A part of periodic table is shown in the following figure Will elem...
Text Solution
|
- A part of periodic table is shown in the following figure Arrange el...
Text Solution
|
- A part of periodic table is shown in the following figure What is t...
Text Solution
|
- A part of periodic table is shown in the following figure Name any t...
Text Solution
|
- Study the below given periodic table in which four elements are indica...
Text Solution
|
- Study the below given periodic table in which four elements are indica...
Text Solution
|
- Study the below given periodic table in which four elements are indica...
Text Solution
|
- A scientist studying reactions of metals and non-metals. He knew group...
Text Solution
|
- A scientist studying reactions of metals and non-metals. He knew group...
Text Solution
|
- A scientist studying reactions of metals and non-metals. He knew group...
Text Solution
|
- A scientist studying reactions of metals and non-metals. He knew group...
Text Solution
|
- A scientist studying reactions of metals and non-metals. He knew group...
Text Solution
|
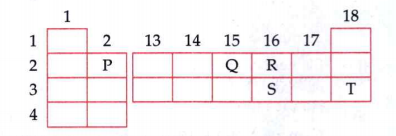 What is the number of electrons in L shell of element T?
What is the number of electrons in L shell of element T?