Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CHETANA PUBLICATION-Periodic Classifications of ELements-EXAMPLE
- The following table shows the position of six elements A,B,C,D,E and F...
Text Solution
|
- Find the odd man out. Lithium,beryllium,boron,Chlorine
Text Solution
|
- Find the odd man out. Sodium,Lithium,Copper,Beryllium
Text Solution
|
- Find the odd man out. Dalton,Dobereiner,Moseley,Newlands
Text Solution
|
- Find the odd man out. boron,Silicon,potassium,antimony
Text Solution
|
- Find the odd man out. Aluminium,Argon,Xenon,Sodium
Text Solution
|
- Find the odd man out. boron,Silicon,potassium,antimony
Text Solution
|
- Find the odd man out. Lithium,beryllium,boron,Chlorine
Text Solution
|
- Complete the anology Dobereiner:Traid::Newlands:
Text Solution
|
- Complete the anology Mendeleev's periodic Table: Atomic mass:: Mode...
Text Solution
|
- Complete the anology Hydrogen:First period ::Lithium:
Text Solution
|
- Complete the anology Fluorine:2,7:: chlorine:
Text Solution
|
- Complete the anology Group 1:Alkali metals :: : alkaline earth meta...
Text Solution
|
- Complete the anology Transition elements :d-block:: inner transitio...
Text Solution
|
- Complete the anology Tellurium:--------:: Radium:Metal
Text Solution
|
- Complete the anology Transition elements:------Inner transition ele...
Text Solution
|
- Complete the anology Lanthanides:Ce to Lu::Actinides:
Text Solution
|
- Complete the anology Ca:Alkaline earth metal:: Cs:
Text Solution
|
- Complete the anology Fe:Electropositive :: CI:
Text Solution
|
- Complete the anology (Li,Na,K):::(F,CI,Br): Group 17
Text Solution
|
- Complete the anology Valency of Na(2,8,1):1(One)::Valency of P(2,8,...
Text Solution
|
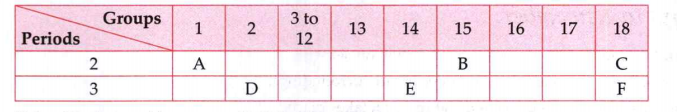 Using the above table , answer the following questions:
Using the above table , answer the following questions: