MAXIMUM PUBLICATION-CLASSIFICATION OF ELEMENTS & PERIODICITY IN PROPERTIES-EXAMPLE
- In transition elements the incoming electron occupies (n-1)d sublevel ...
Text Solution
|
- The arguments made by two students are as given: Student 1 : 'Hydrogen...
Text Solution
|
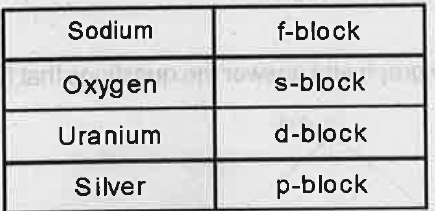
- Match the following:
Text Solution
|
- a) Which one has greater size, Na or K? b) Justify your answer.
Text Solution
|
- The general cheracteristics of a particular block of elements is as gi...
Text Solution
|
- The variation in electron gain enthalpies of elements is less systemat...
Text Solution
|
- Some elementS are given. Li, Cs, Be, C, N, O F, l, Ne, Xe. a) Arrange...
Text Solution
|
- a) What is meant by atomic radius? b) Explain covalent radius. c) Wr...
Text Solution
|
- Consider the statement: 'The element with 1s^2 configuration belongs t...
Text Solution
|
- The properties of elements are a periodic function of their atomic wei...
Text Solution
|
- a) Define ionisation enthalpy. b) IE1< IE2 < IE3 What is meant by th...
Text Solution
|
- Say whether the following are true or false: a) On moving across a pe...
Text Solution
|
- a) On moving down a group what happens to electron gain enthalpy? b) ...
Text Solution
|
- Electron gain enthalpy is an important periodic property. a) What is m...
Text Solution
|
- a) The electron gain enthalpies of Be and Mg are positiVe. What is you...
Text Solution
|
- During a group discussion a student argued that ionization enthalpy de...
Text Solution
|
- a) Which of the following has higher first ionization enthalpy, N or O...
Text Solution
|
- Electronegativity differs from electron gain enthalpy. a) Do you agre...
Text Solution
|
- Ionization enthalpy is an important periodic property. a) What is the...
Text Solution
|
- The second period elements show anomalous behaviour. a) Give reason. ...
Text Solution
|