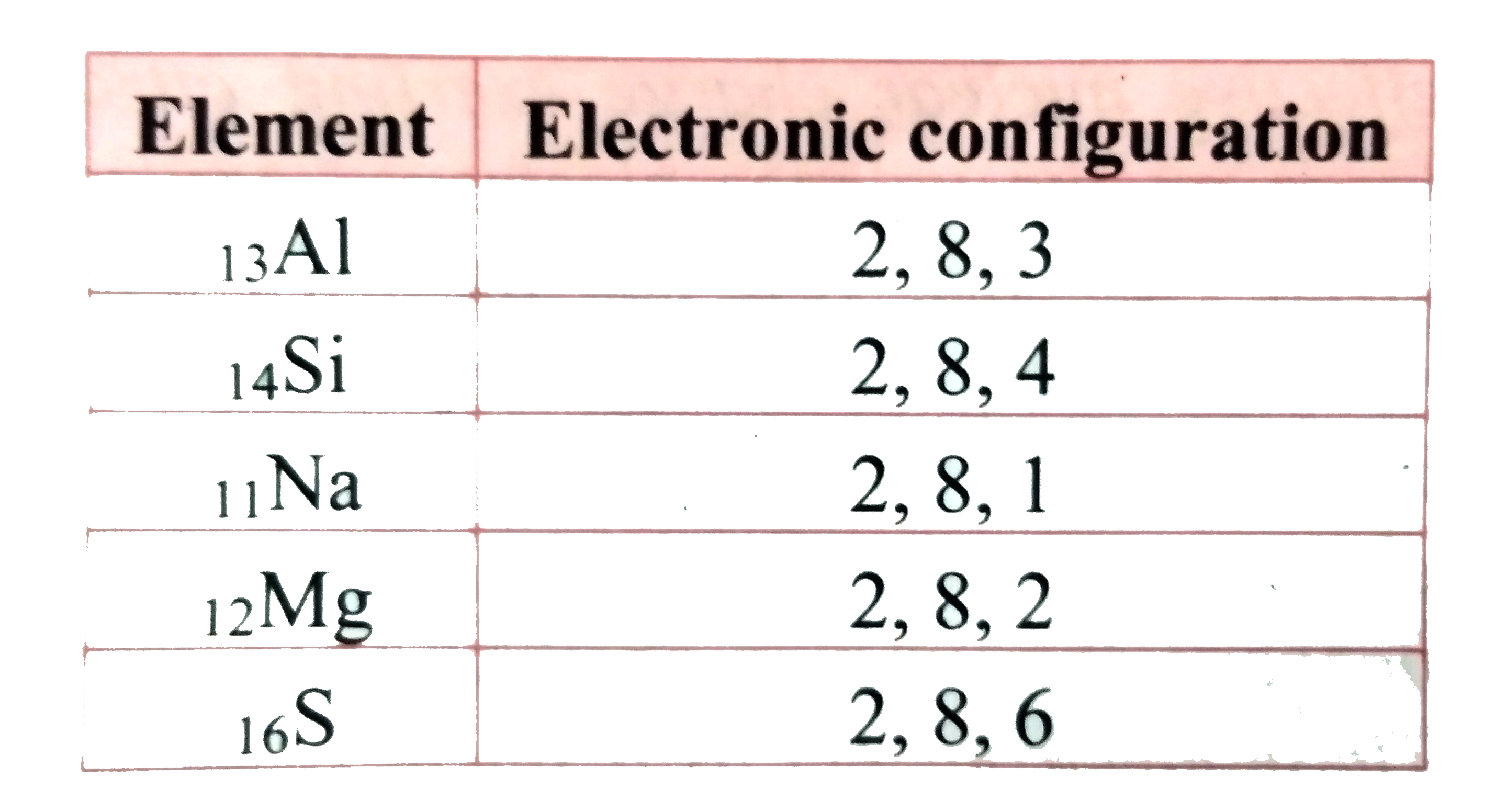
Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Give reasons|5 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Distinguish between|2 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Match the following|4 VideosMETALLURGY
TARGET PUBLICATION|Exercise CHAPTER ASSESSMENT|12 Videos
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -Answer the following
- The atom having the smallest atomic mass .
Text Solution
|
- The most electronegative atom
Text Solution
|
- The noble gas with the smallest atomic radius
Text Solution
|
- The most reactive nonmetal
Text Solution
|
- An element has its electronic configuration as (2,8,2). Now answer the...
Text Solution
|
- An element has its electronic configuration as (2,8,2). Now answer the...
Text Solution
|
- An element has its electronic configuration as (2,8,2). Now answer the...
Text Solution
|
- An element has its electronic configuration as (2,8,2). Now answer the...
Text Solution
|
- What is the difference between electropositivity and electronegativity...
Text Solution
|
- Consider the elements of period 2 in the modern periodic table. Answer...
Text Solution
|
- Consider the elements of period 2 in the modern periodic table. Answer...
Text Solution
|
- Consider the elements of period 2 in the modern periodic table. Answer...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|
- Write down the electronic configuration of the following elements from...
Text Solution
|