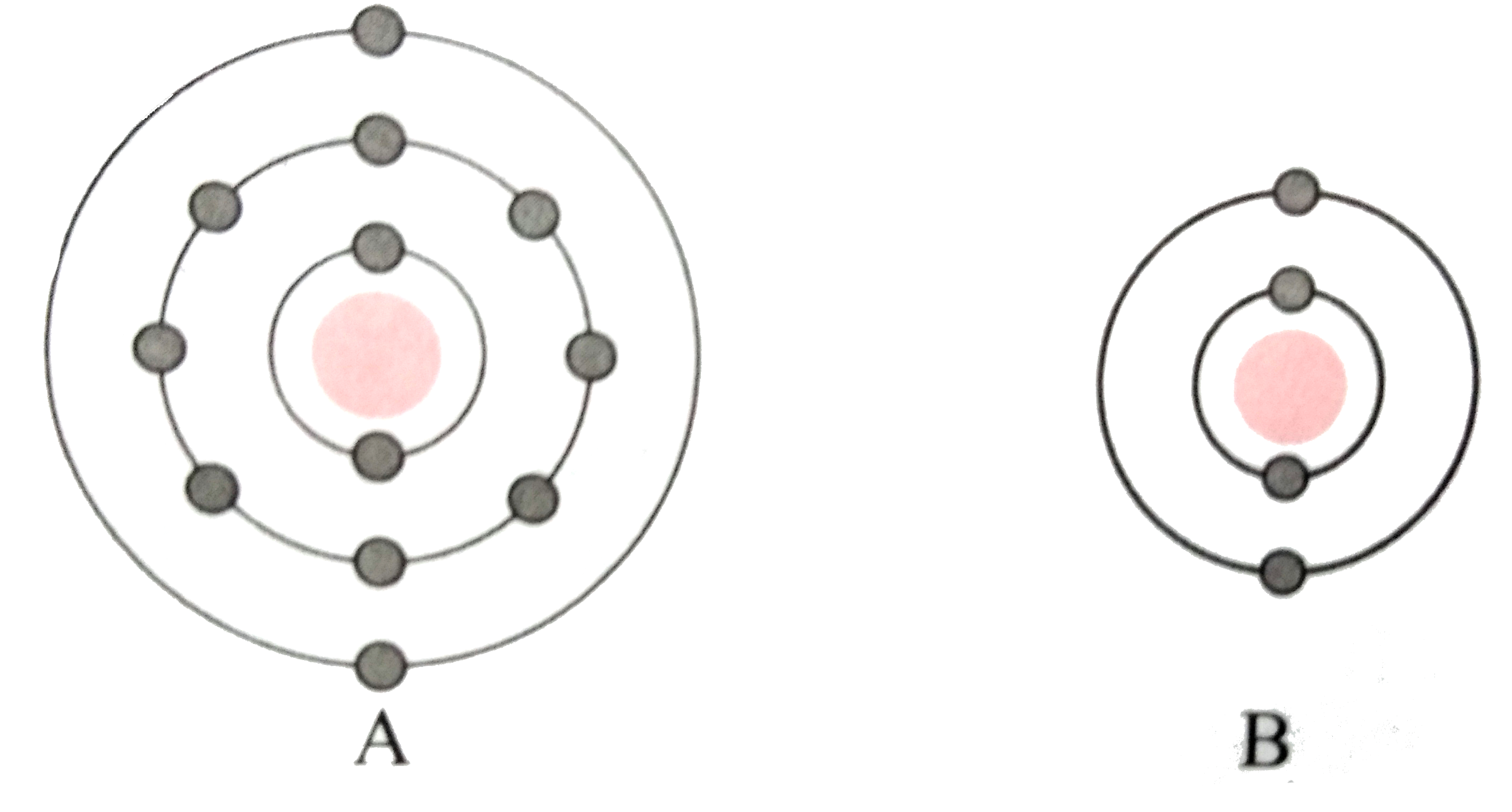
Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Questions based on paragraph|15 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Apply your knowledge|49 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Complete the given chart/table|2 VideosMETALLURGY
TARGET PUBLICATION|Exercise CHAPTER ASSESSMENT|12 Videos
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS -Questions based on diagram
- Draw electronic configuration diagram for: Potassium
Text Solution
|
- Draw electronic configuration diagram for: Argon
Text Solution
|
- Find out the number of valence electrons and valency of the atoms repr...
Text Solution
|
- Find out the number of valence electrons and valency of the atoms repr...
Text Solution
|
- Atoms of two different elements are represented in the following diagr...
Text Solution
|
- Atoms of two different elements are represented in the following diagr...
Text Solution
|
- Atoms of two different elements are represented in the following diagr...
Text Solution
|
- Study the following periodic table. A student has marked two periodic ...
Text Solution
|
- Observe the following diagram and write the answers of the following q...
Text Solution
|
- Observe the following diagram and write the answers of the following q...
Text Solution
|
- Observe the following diagram and write the answers of the following q...
Text Solution
|
- Observe the following diagram and write the answers of the following q...
Text Solution
|
- Observe the following diagram and write the answers of the following q...
Text Solution
|
- Study the below given periodic table in which four elements are indica...
Text Solution
|
- Study the below given periodic table in which four elements are indica...
Text Solution
|
- Study the below given periodic table in which four elements are indica...
Text Solution
|