Text Solution
Verified by Experts
Topper's Solved these Questions
METALLURGY
TARGET PUBLICATION|Exercise QUESTION BASED ON PARAGRAPH|5 VideosMETALLURGY
TARGET PUBLICATION|Exercise APPLY YOUR KNOWLEDGE|26 VideosMETALLURGY
TARGET PUBLICATION|Exercise COMPLETE THE GIVEN CHART/TABLE|2 VideosCHEMICAL REACTIONS AND EQUATIONS
TARGET PUBLICATION|Exercise Chapter Assessment|31 VideosPERIODIC CLASSIFICATION OF ELEMENTS
TARGET PUBLICATION|Exercise Chapter Assessment|16 Videos
Similar Questions
Explore conceptually related problems
TARGET PUBLICATION-METALLURGY-QUESTION BASED ON DIAGRAM
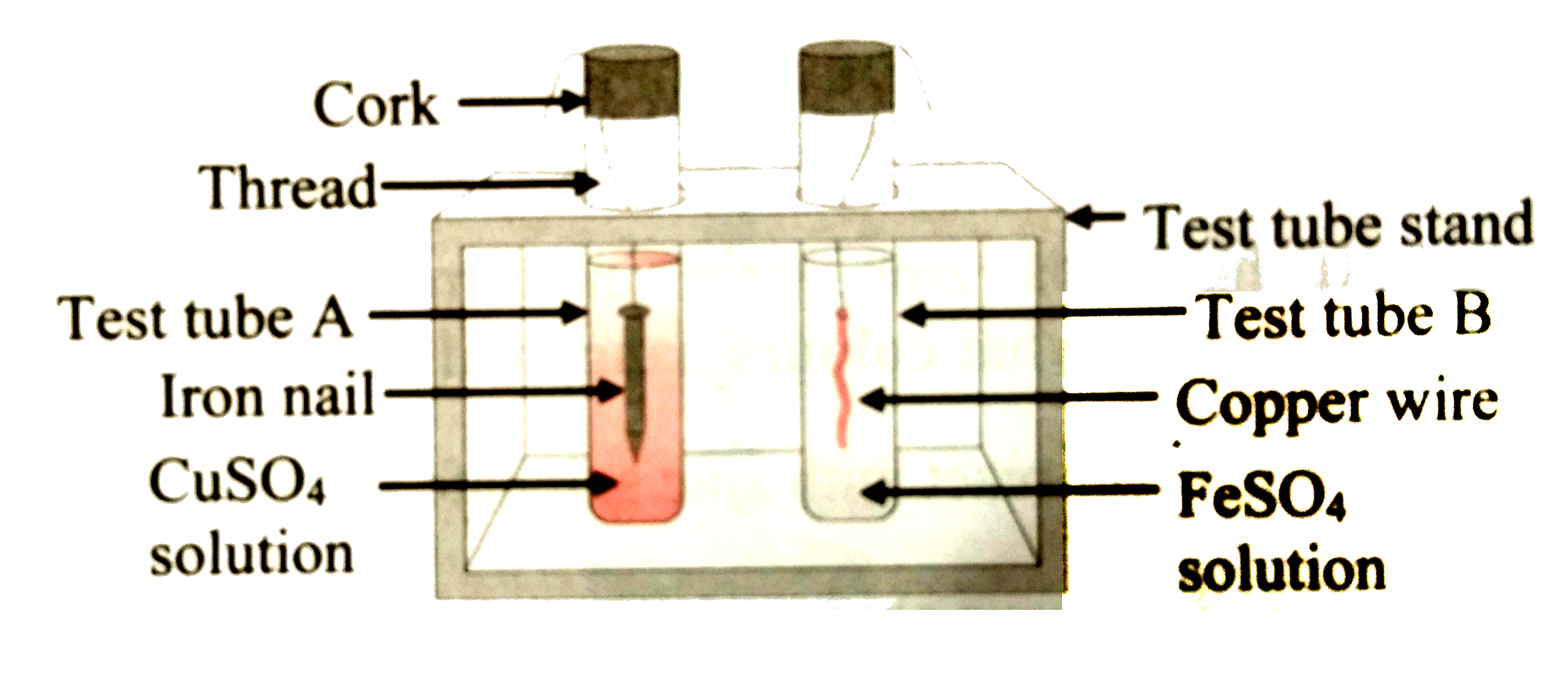
- Study the following experimental set-up and answer the following quest...
Text Solution
|
- Study the following experimental set-up and answer the following quest...
Text Solution
|
- Study the following experimental set-up and answer the following quest...
Text Solution
|
- In the given reactivity series, some metals are misplaced. Rearrange t...
Text Solution
|
- Draw a neat and labelled diagram Magnetic separation method
Text Solution
|
- Draw a neat and labelled diagram Froth floatation method
Text Solution
|
- Draw a neat and labelled diagram Electrolytic reduction of alumina
Text Solution
|
- Draw a neat and labelled diagram Hydraulic separation
Text Solution
|
- Identify the process represented by the following diagram. Describe th...
Text Solution
|
- Study the diagram and answer the following questions What does ...
Text Solution
|
- Study the diagram and answer the following questions Write the ...
Text Solution
|