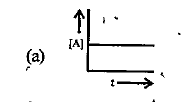
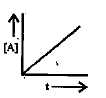
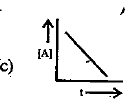
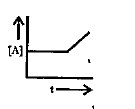
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-CHEMICAL KINETICS-QUESTION BANK
- The rate constant is given by the equation K=Ae^(-Ea//RT)which factor ...
Text Solution
|
- For the reaction ,H2(g)+Br(g)=2HBr(g),the reaction rate=K[H2][Br2]^(1/...
Text Solution
|
- A zero order reaction is one
Text Solution
|
- Rate equation for a second order reaction is :
Text Solution
|
- In Arrhenius equation K=Ae^-Ea//RT ,the quantity-Ea//RT is referred as...
Text Solution
|
- The temperature coefficient of most of the reaction lies between:
Text Solution
|
- Which one is unimolecular reaction :
Text Solution
|
- Rate equation is the expression that gives the relation between rate o...
Text Solution
|
- A 1st order reaction has K value 1.5xx10^(-6) at 200^@C. The reaction ...
Text Solution
|
- Thermal decomposition of a compound is of first order. If 50% of a sam...
Text Solution
|
- Prove that time required for the completion of 3/4 of reaction of the ...
Text Solution
|
- The half life period of 1st order reaction of A is 2 minutes. How long...
Text Solution
|
- Define rate of reaction .
Text Solution
|
- Define order of reaction.
Text Solution
|
- Explain molecularity of a reaction.
Text Solution
|
- Calculate the unit of 1st order rate constant
Text Solution
|
- Give one example of unimolecular reaction.
Text Solution
|
- Give one example of bimolecular reaction.
Text Solution
|
- Write relationship between the rate constant and its activation energy...
Text Solution
|
- The minimum energy which molecules need to acquire before they can rea...
Text Solution
|