Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CHETANA PUBLICATION-Biomolecules-EXERCISE
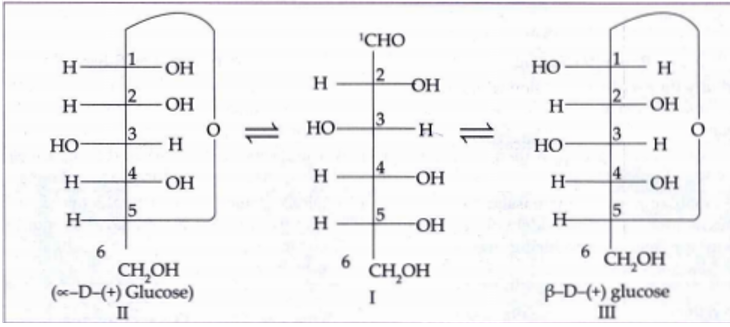
- In fig. 14.2 which carbon is called the anomeric carbon?
Text Solution
|
- CH2OH-CO-(CHOH)4-CH2OH is an example of
Text Solution
|
- Open chain formula of glucose does not contain
Text Solution
|
- Which of the following does not apply to CH2NH2-COOH
Text Solution
|
- Tryptophan is called essential amino acid because
Text Solution
|
- A disulfide link gives rise to the following structure of protein.
Text Solution
|
- RNA has
Text Solution
|
- What is the type of ring structure in glucose?
Text Solution
|
- What is a glycosidic linkage?
Text Solution
|
- Which sugar is used as sweetener in bakery and confectionery products?
Text Solution
|
- Which is the storage carbohydrate of plants?
Text Solution
|
- Which is the storage carbohydrate of animals?
Text Solution
|
- Which are the fundamental structural materials of animal bodies?
Text Solution
|
- The enzyme secreted by the pancreas is
Text Solution
|
- The enzyme which controls blood sugar is
Text Solution
|
- The enzyme present in saliva and hydrolyses starch is
Text Solution
|
- Milk sugar is
Text Solution
|
- The store house for all biological information is
Text Solution
|
- Raffinose is a
Text Solution
|
- A biological catalyst is essentially .
Text Solution
|
- Which of the following is NOT a constituent of RNA?
Text Solution
|