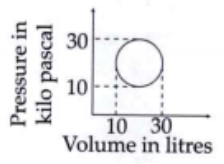
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CHETANA PUBLICATION-Thermodynamics-EXERCISE
- If w1 and w2 are the amounts of work done in the given two indicator d...
Text Solution
|
- In the indicator diagram shown, the work done along the path AB is br>
Text Solution
|
- In the indicator diagram shown, the work done in the cyclic process is...
Text Solution
|
- The internal energy of air in a room of volume 40 m^3 atstandard atmos...
Text Solution
|
- A gas undergoes a change of state during which 100 J of heat is suppli...
Text Solution
|
- The amount of work done to increase the temperature of one mole of an ...
Text Solution
|
- Two engines A and B have their sources at 400 K and 350 K and sinks at...
Text Solution
|
- If two thirds of the heat taken by a heat engine from the source is gi...
Text Solution
|
- The efficiency of a heat engine is 20%, when the working substance is ...
Text Solution
|
- If the absolute temperatures of the source and sink of a heat engine a...
Text Solution
|
- Two Carnot engines A and B are operated in series. A receives heat at ...
Text Solution
|
- A refrigerator has a coefficient of performance 9. If the surrounding ...
Text Solution
|
- A refrigerator works between 0^@C and 27^@C. If heat is to be removed ...
Text Solution
|
- A gas in a closed container is heated with 10 J of energy , causing t...
Text Solution
|
- Which of the following is an example of the first law of thermodynamic...
Text Solution
|
- Efficiency of a Carnot engine is large when
Text Solution
|
- The second law of thermpdynamics deals with transfer of
Text Solution
|
- During refrigration cycle, heat is rejected by the refrigerant in the
Text Solution
|
- Select and write correct alternative from the following alternatives: ...
Text Solution
|
- State first law of thermodynamics.
Text Solution
|