A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD PUBLICATION-ELECTROCHEMISTRY-QUESTION BANK
- Of the following matals that cannot be obtained by electrolysis of the...
Text Solution
|
- A certain metal fails to liberate H2 gas from a moderately conc. HCI s...
Text Solution
|
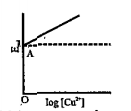
- Cu^(2+) +2e rarr Cu, log [Cu^(2+)] vs E(red).graph is of the type as s...
Text Solution
|
- For which cell emf is independent of the concentration of electrolyte...
Text Solution
|
- The reaction, Cu^(2+)(aq) +2CI^- (aq) rarr Cu(s) +CI2(g) has E(cell)^...
Text Solution
|
- For the electrochemical cell,M[M^+]X^-|X,E^@(M+//M)=0.44 V and E(X//X...
Text Solution
|
- Electrolytic reduction of alumina to aluminium by Hall-Heroult process...
Text Solution
|
- When 9.65 coulomb of electricity is passed through a solution of AgNO3...
Text Solution
|
- Standard electrode potentials of Fe^(2+) +2e rarrFe and Fe^(3+)+3e ra...
Text Solution
|
- I mole of AI is deposited by X coulomb of electricity passing through ...
Text Solution
|
- Copper from copper sulphate solution can be displaced by ………….The stan...
Text Solution
|
- The oxidation potential of a hydrogen electrode at pH =10 and P(H2) = ...
Text Solution
|
- The number of Faraday required to gneerate 1g of Mg from MgCl2 is:
Text Solution
|
- emf of cell Ni, Ni^(2+)(1.0M) || Au^(3+)(1.0M), Au is …………If E^@ for N...
Text Solution
|
- E^@ for the half cell reactions are as, Zn→Zn^(2+) +2e,E^@=+0.76V Fe→F...
Text Solution
|
- A certaom cirrent liberates 0.504 g of hydrogen in 2 hr. How many gram...
Text Solution
|
- The standard reduction electrode potentials of your metals A,B, C and ...
Text Solution
|
- An apparatus used for the measurement of quantity of electricity is kn...
Text Solution
|
- The amount of an ion descharged during electrolyses is not dependent o...
Text Solution
|
- If an iron rod is deppedin CuSO4 solution:
Text Solution
|